Hylleraas Helium - starting Many Electron Atoms
More advanced variational methods - the functions are not product
functions;
NOT independent particles;
 g1.png
g1.png
Hylleraas function for helium. Hylleraas was a Norwegian physicist
(professor in Oslo,
in 1930-1937 at Christian Michelsen Institute in Bergen). Note that the
trial functions of Hylleraas
type are not product functions.
Therefore
they automatically include electron-electron
correlations.
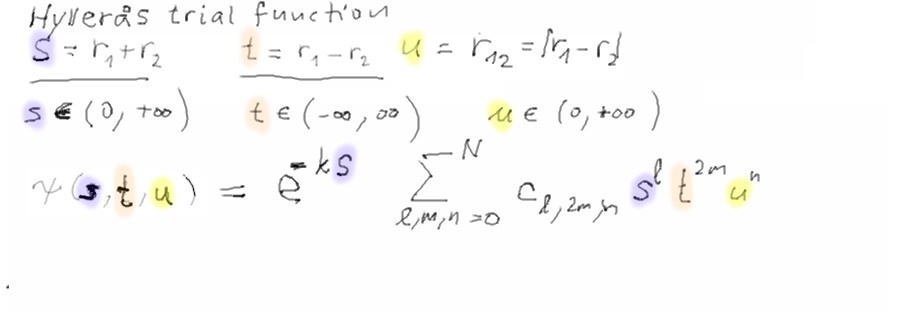 g2.png
g2.png
Other types of correlated functions can be much more complicated.
 g3.png
g3.png
Why are the precise functions of interest - for example they allow the
possibility
of looking for new phenomena.
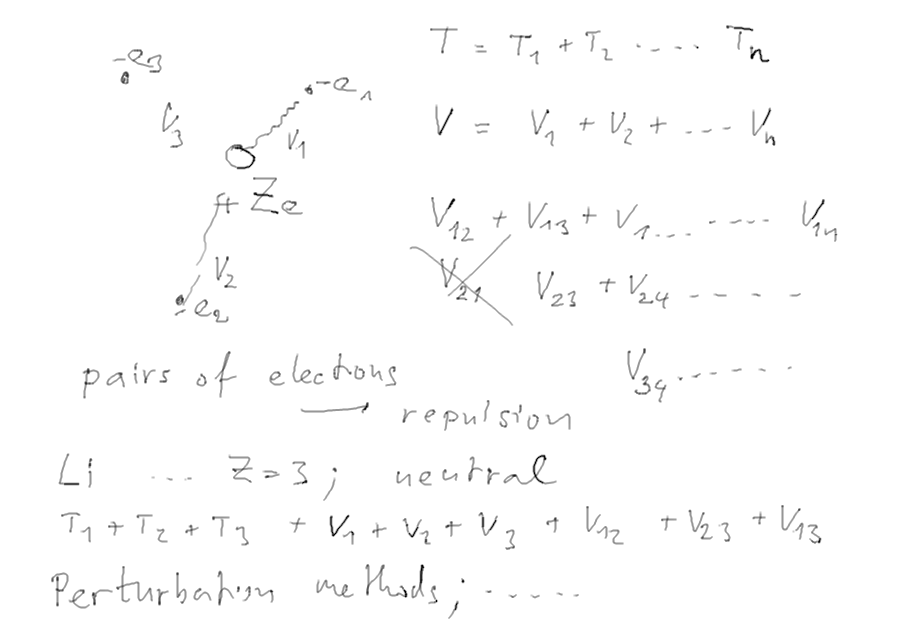
Starting the
Many-electron atoms
 g4.png
g4.png
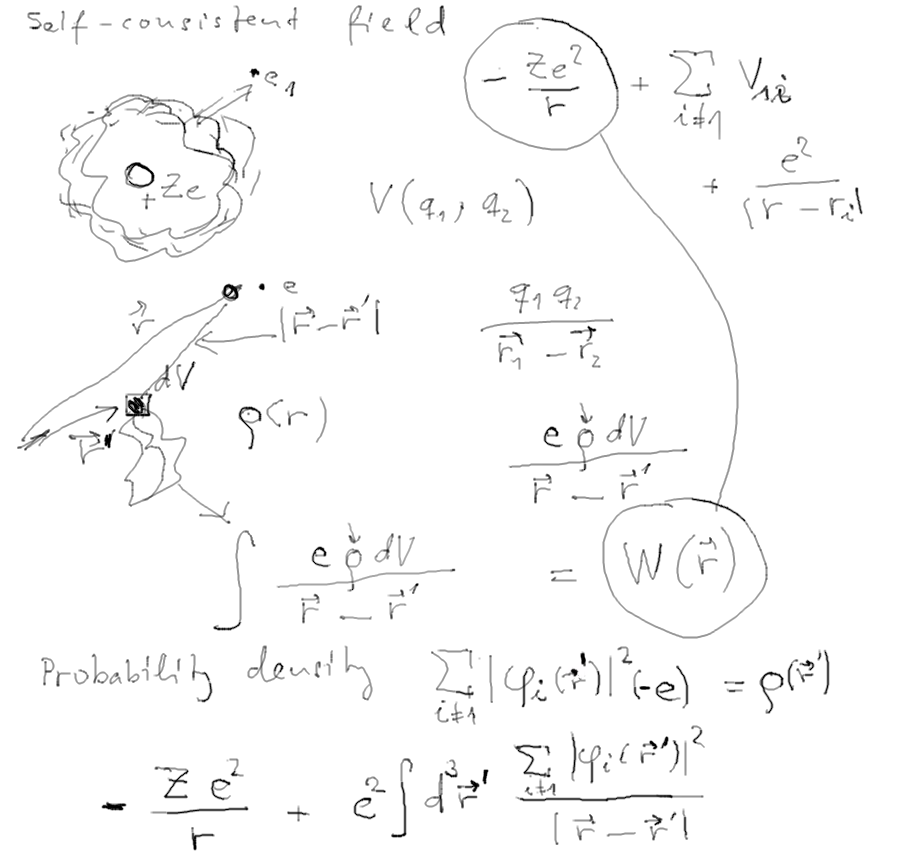
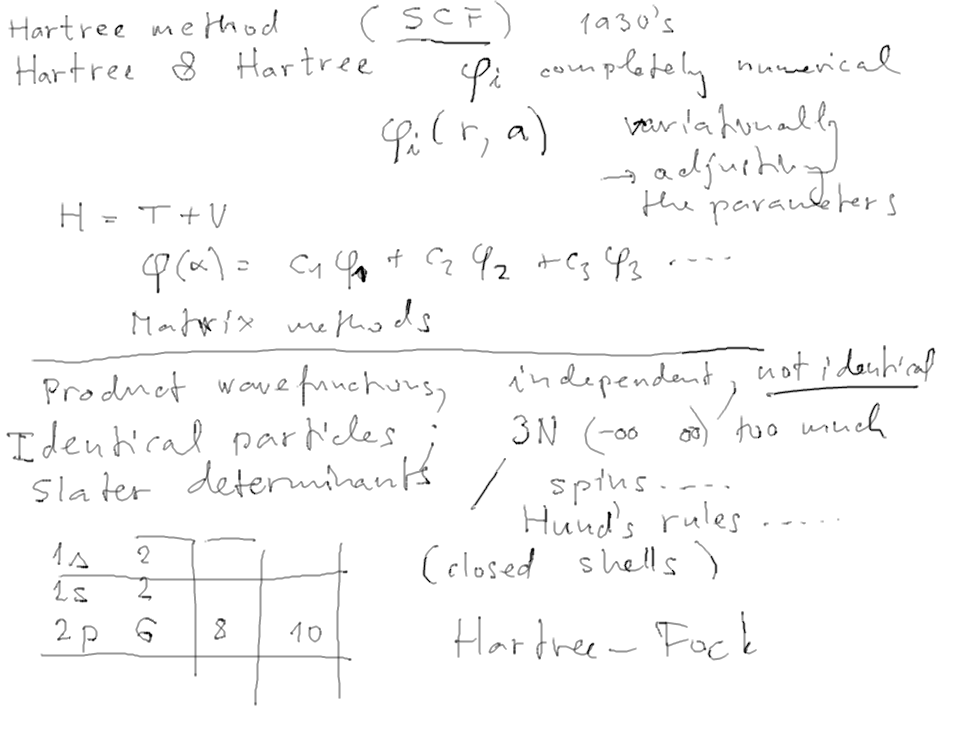
The concept of Self-consistent
field - SCF
known also from theretical chemistry;
also used in nuclear physics
Interaction of an electron with a cloud of charge (the other electrons)
 g5.png
g5.png
Self-consistent field in an ITERATIVE PROCESS
 g6.png
g6.png
Trial functions type (0)
-> electron densities type (0)
-> potential for cloud interaction W(0)
T+V+W(0) -> solutions give functions type (1)
-> electron densities type (1)
-> potential for cloud interaction W(1)
T+V+W(1) -> solutions give functions type (2)
-> electron densities type (2)
-> potential for cloud
interaction W(2)
T+V+W(2) -> solutions give functions
type (3)
-> electron densities type (3)
-> potential for cloud
interaction W(3)
T+V+W(3) -> solutions give functions
type (4)
-> electron densities type (4)
-> potential for cloud
interaction W(4)
..........
T+V+W(n) -> solutions give functions type
(n+1)
-> electron densities type (n+1)
-> potential for cloud
interaction W(n+1)
stop when
W(n) = W(n+1)
- the potentials, functions, are self-consistent
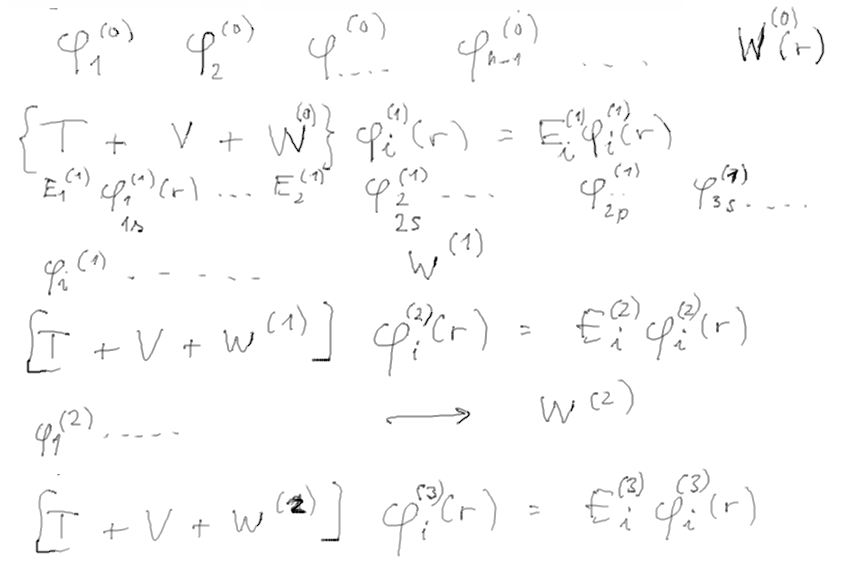 g7.png
g7.png
 g8.png
g8.png
The Hartree method (schematically)
 g9.png
g9.png
 g1.png
g1.png 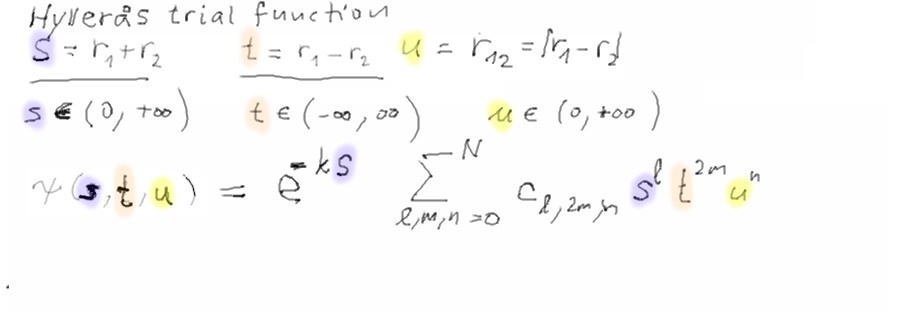 g2.png
g2.png  g3.png
g3.png  g4.png
g4.png  g5.png
g5.png  g6.png
g6.png 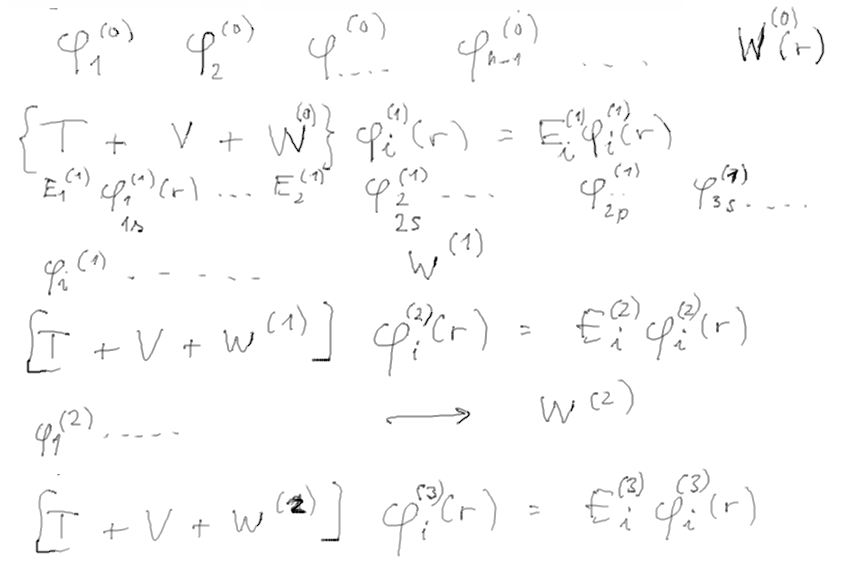 g7.png
g7.png  g8.png
g8.png  g9.png
g9.png