Files to work on Self-consistent field program ( FORTRAN, 1963 Herman & Skillman )
Fortran code SCF-work - Hartree (1995 Stockholm Course)Mac and Windows - executable code in the above zip-file |
||||||
| Unix-Linux Users (including Mac Users
- g95 for Mac available) Use the herman3.f and radhyd3.f FORTRAN SOURCES compile by gfortran -o herman3 herman3.f ( replace gfortran by the compiler you have, e.g. g95 g77 ...... fortran ..... ) and execute as described in the course SCF-work - Hartree (1995 Stockholm Course) but nowadays with ./herman3 < in13 ( dot slash before the code name; security is the reason .... ) |
||||||
| on
windows cd D:\some_data\your_names\fortran3 (or where is your working directory wiyh fortran3 herman3 < in13 in the com.exe window (cd to the correct directory first ) |
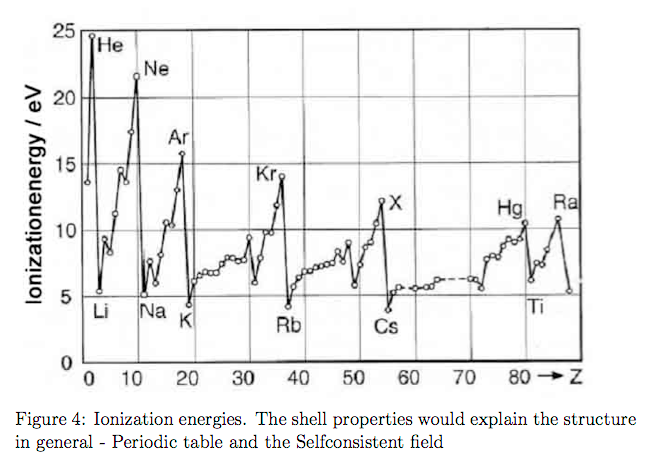 0-Ionization-potentials.png
0-Ionization-potentials.png 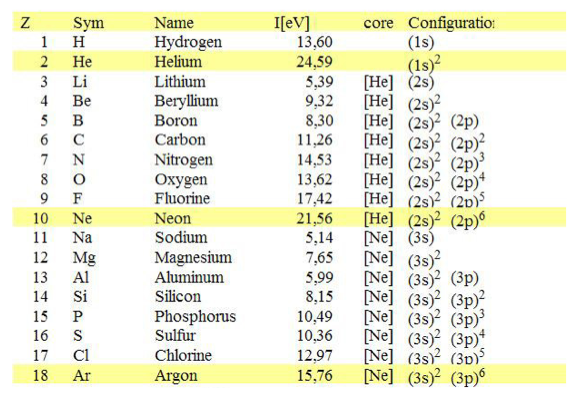 1-Z=1_to_Z=18.png
1-Z=1_to_Z=18.png 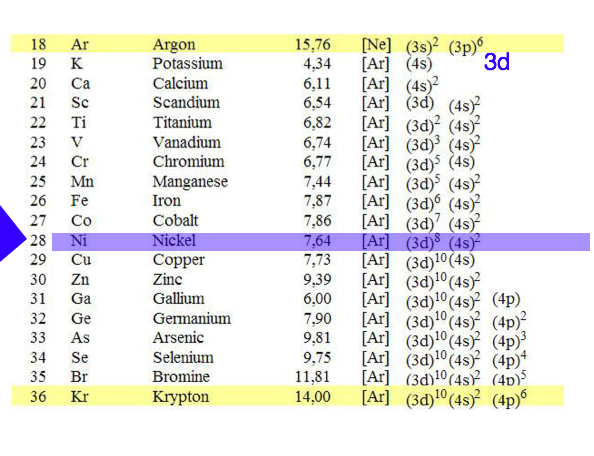 2-Z=18_to_Z=36.png
2-Z=18_to_Z=36.png 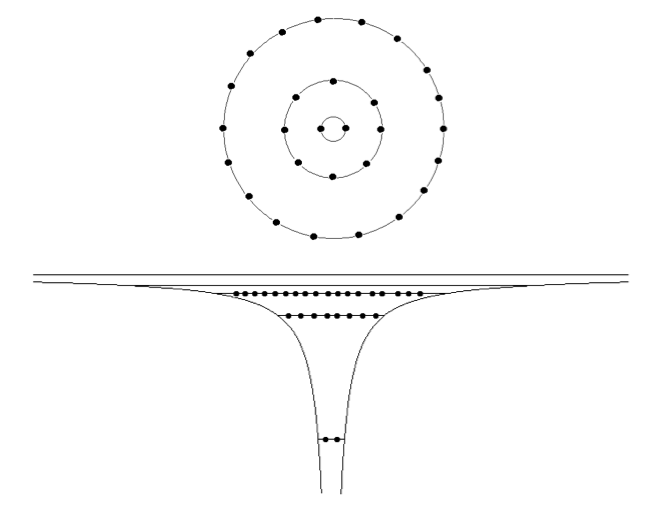 3-Aufbau_Principle.png
3-Aufbau_Principle.png 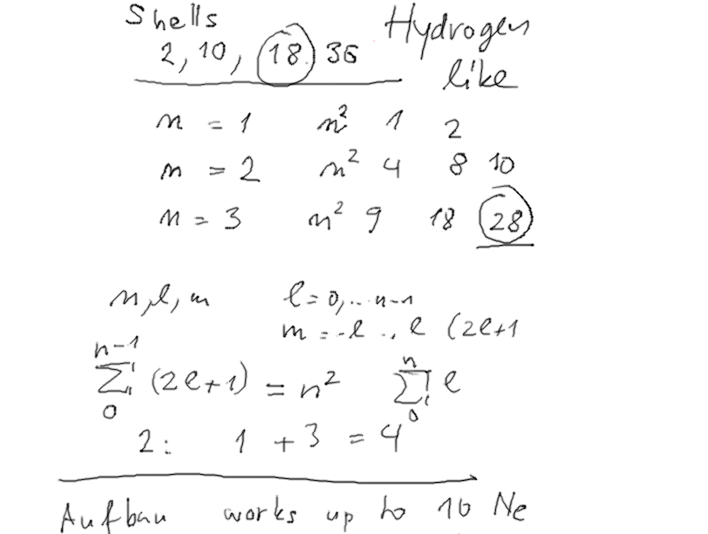 3-magic-numbers_NxN.png
3-magic-numbers_NxN.png 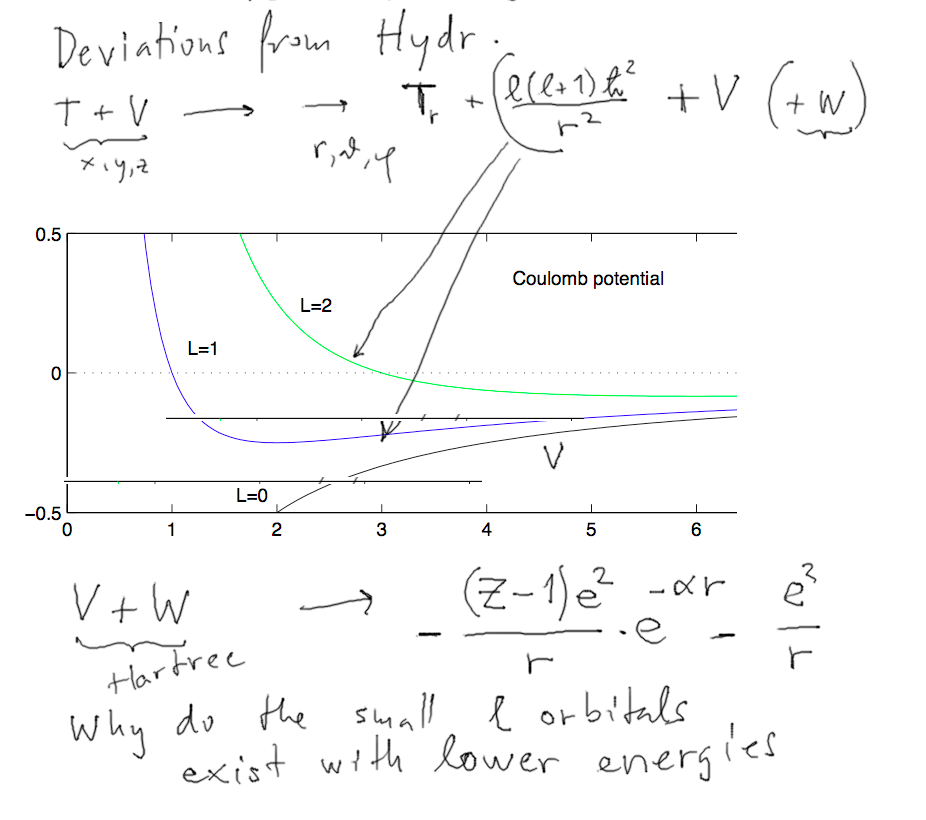 4-explain-why-centrifugal.png
4-explain-why-centrifugal.png
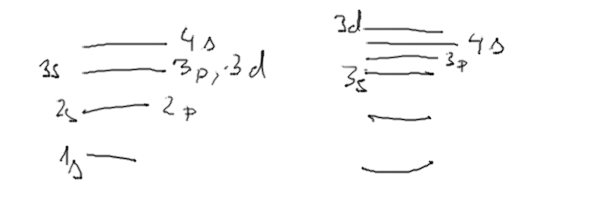 5__1s_2s_2p_3s_3p_4s_3d.png
5__1s_2s_2p_3s_3p_4s_3d.png 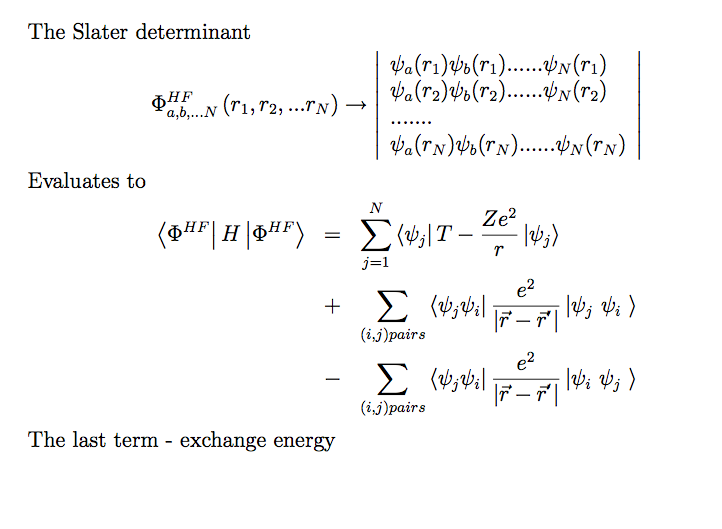 6-Slater_determinant.png
6-Slater_determinant.png 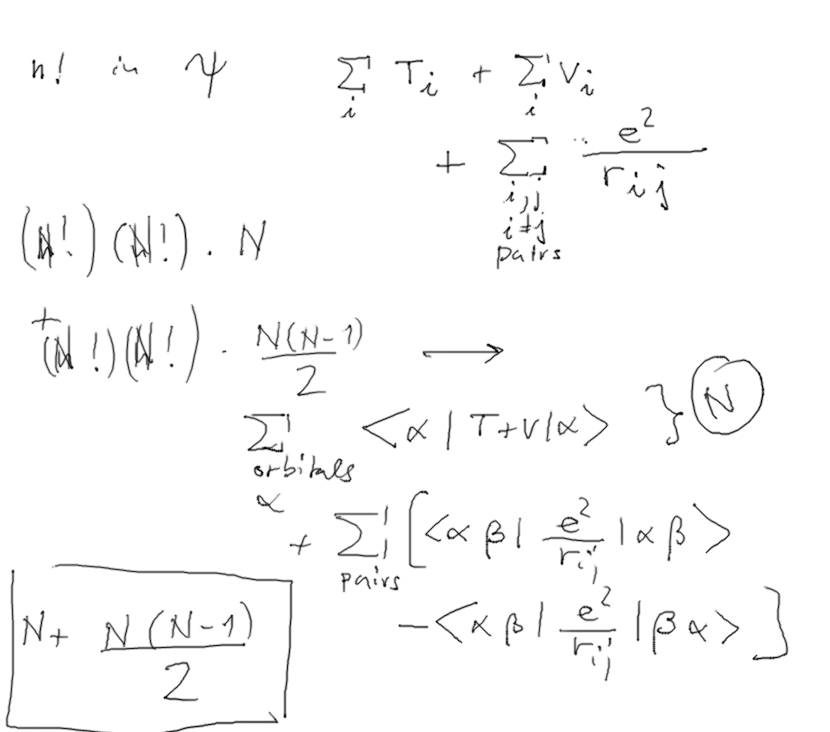 7-Energy_in_Slater_determinant.png
7-Energy_in_Slater_determinant.png 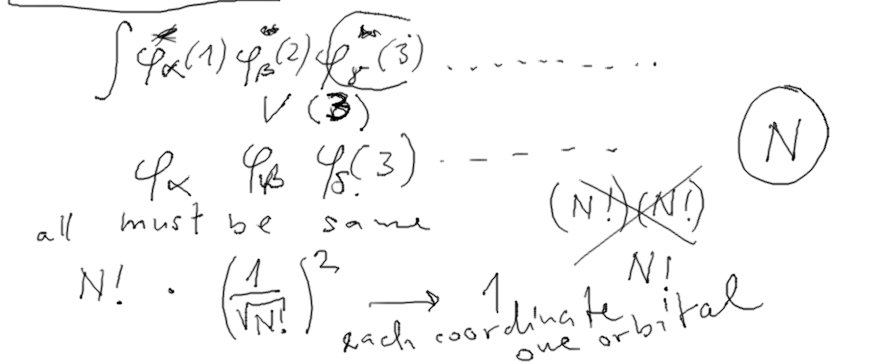 8-counting.png
8-counting.png  9-Result_Slater.png
9-Result_Slater.png 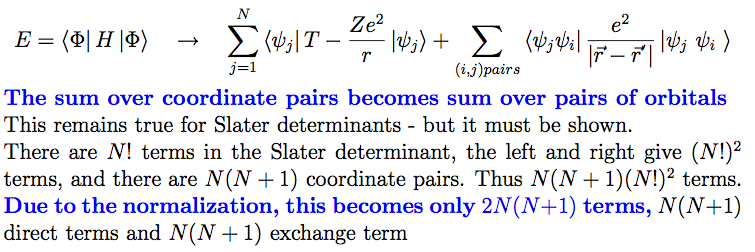 9b-Result_Slater.png
9b-Result_Slater.png 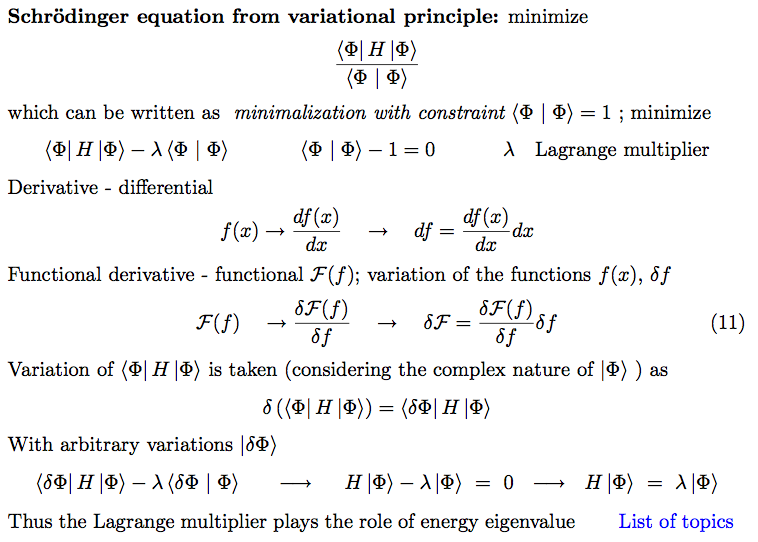 a-Schrodinger.png
a-Schrodinger.png  b-variation-Schrodinger.png
b-variation-Schrodinger.png 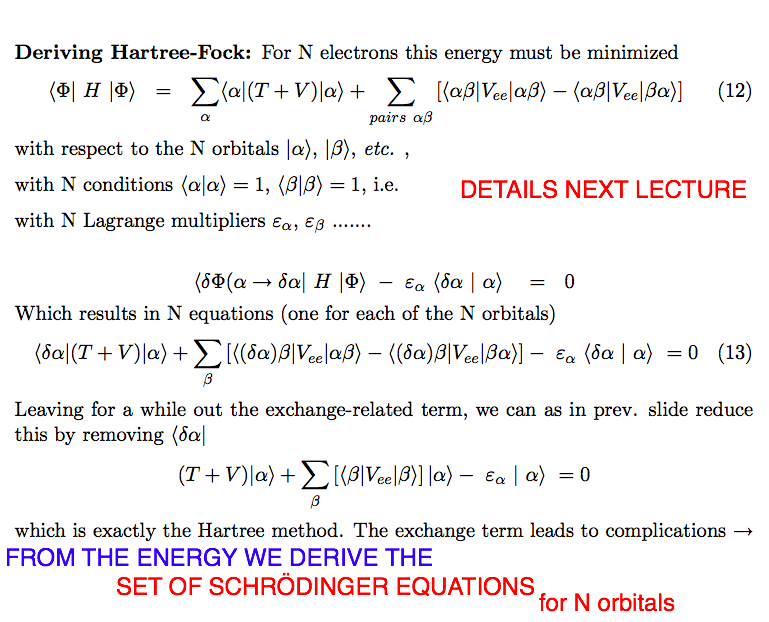 b-variation-HARTREE.png
b-variation-HARTREE.png