Perturbation theory - repulsion. Variation Method: Compare with
Experiment
The 2-electron hamiltonian consists of two single-electron parts for
each electron and their interaction - repulsion.
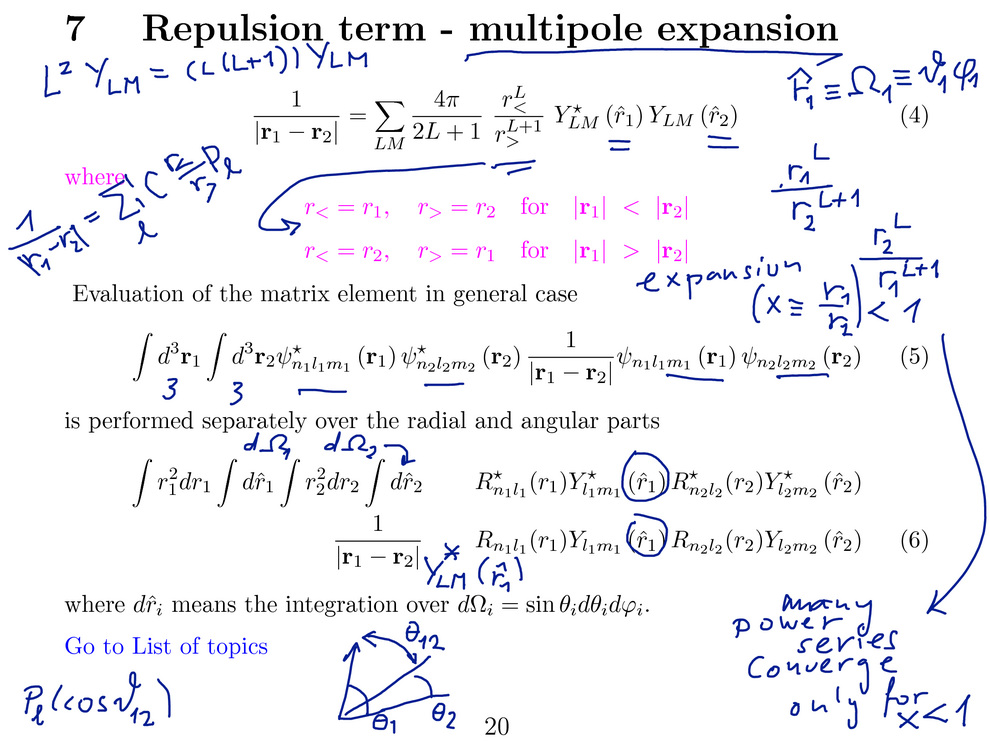
The repulsion term needs to be evaluted as matrix element between the
states of independent electrons.
The MULTIPOLE EXPANSION in terms of spherical functions (and Legendre
polynomials of the angle - see below)
The six-dimensional integrals are transformed to a SUM over terms with
two independent integrals over
each of the pair of angles (theta, phi, collectively Omega or r-hat)
and a two r-variable integral non-separable due to the r-larger --
r-smaller terms)
For ground state (1s)(1s) on "both sides" the matrix element reduces to
one term only
small_0010.png

small_0010.png
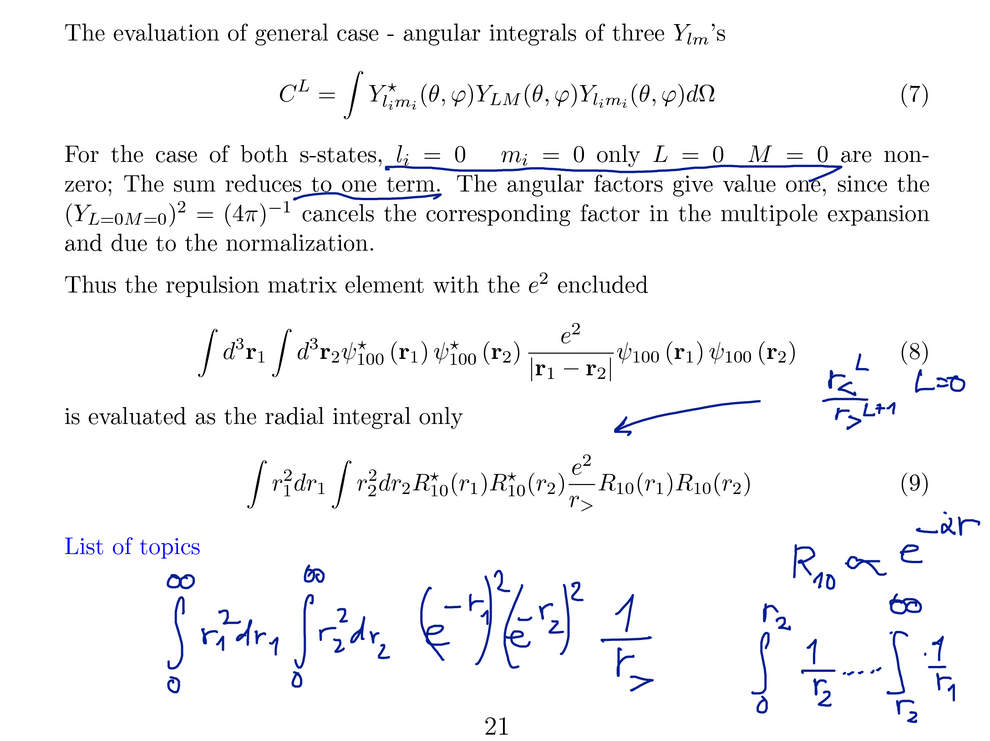
In the last part the general matrix elements for (n, l)(n',l')
- (n'' l'') (n''' l''') is illustrated - some aspects with the
"triangle relation"
Here we worked on the sheets of the presentation - first mentioning the
r-larger r-smaller origin, The Legendre polynomial expansion
The notation
small_0011.png
small_0011.png
Legendre expansion is in terms of
cosine of the angle between the two position vectors
http://en.wikipedia.org/wiki/Legendre_polynomials#Orthogonality
and also in the next section
http://en.wikipedia.org/wiki/Legendre_polynomials#Applications_of_Legendre_polynomials_in_physics
small_0012.png
small_0012.png
FOR THE GROUND STATE
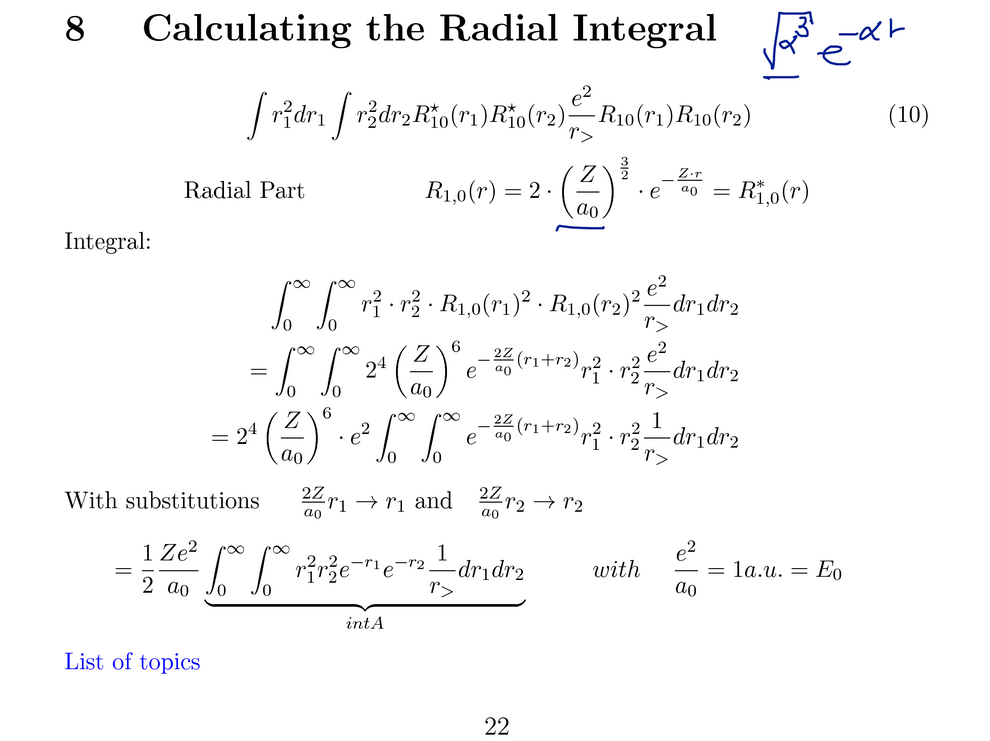
The angular part evaluates to 1, it remains to evaluate the radial
integral. The radial functions are in general
polynomials times an exponential
small_0013.png
small_0013.png
small_0014.png
small_0014.png
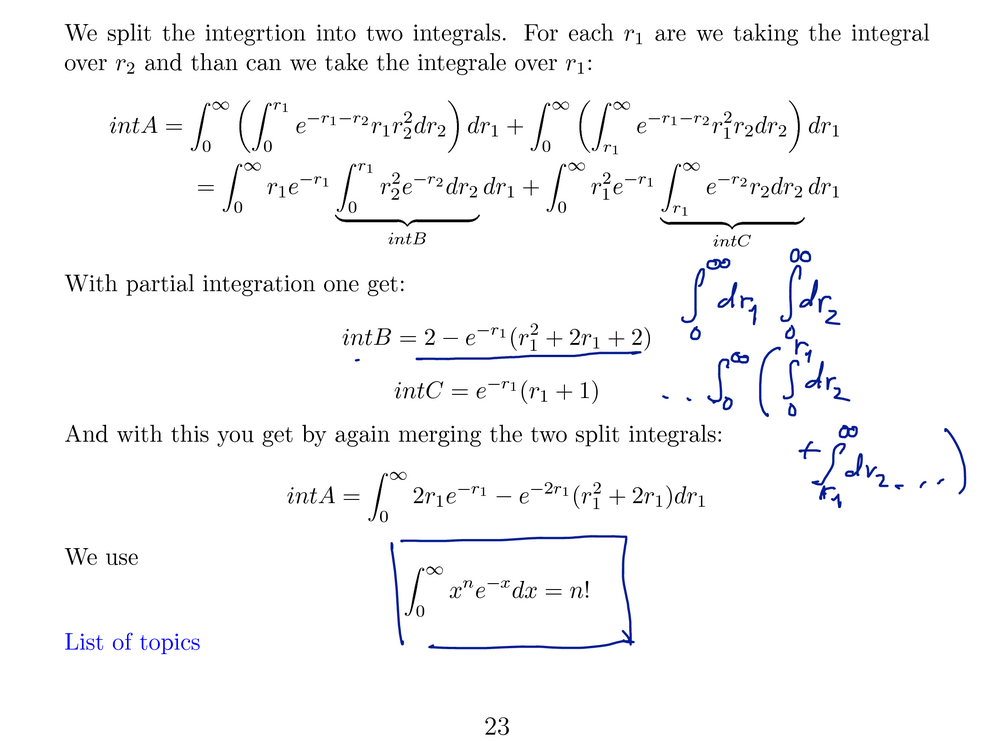
After evaluation by quite a few elementary terms the final result
is obtained - a rather simple expression.
A n important feature - this term depends on Z -linear proportionality.
Thus the repulsion scales with Z
(note that all the single particle terms scale with Z2
)
small_0015.png

small_0015.png
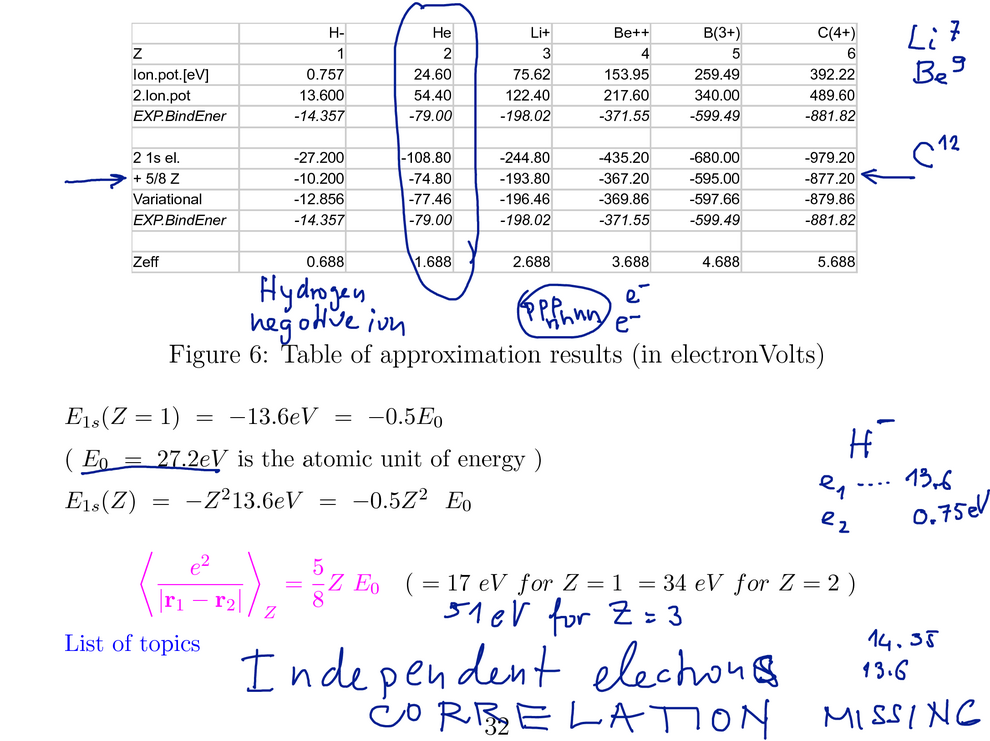
Here we discussed the result - with reference to the table below (there compared with the
variational method)
small_0020.png

small_0020.png
Here in the last part we sketched how the different terms influence the
resulting ground state energy
Experimental value is LOWER than the evaluated - "with perturbation
theory approximate
wavefunction" - see variational theorem
Variational theorem: The ground state energy is smaller or equal to
any approximated function
EXPECTATION VALUE (i.e. that matrix element ... see
the application )
small_0030.png

small_0030.png
VARIATIONAL METHOD - look for a wavefunction which gives lowest
value
of the expectation value
And here we sketch how to look - effective Z - which
we denote z
small_0040.png

small_0040.png
In the lower part of the above - we sketch how we define something to
vary - effective Z - which
we denote z
We evaluate the T and V for each electron and the repulsion -
simply by recognizing how they depend on the
Z-value of the wavefunction. The potential term scales with Z 2
- but the term contains explicitely only one Z.
Thus the dependence will be zZ. The
kinetic energy term scales with Z2
- but contains no explicit Z. Thus it must
depend on z2
. The repulsion also has no Z-dependence explicitely, but was evaluated
to 5/8 Z,
i.e. it must become
... see the next picture ... 5/8 z
With this replacements we are ready to find the "best value" of
effective z
- varying the z and finding the minimum.
I.e. we find the z for which the expression gives a minimum - zero
derivative
(using the variation theorem )
small_0050.png

small_0050.png
(this table was also us ed to
discuss the perturbation result )
small_0051.png
small_0051.png
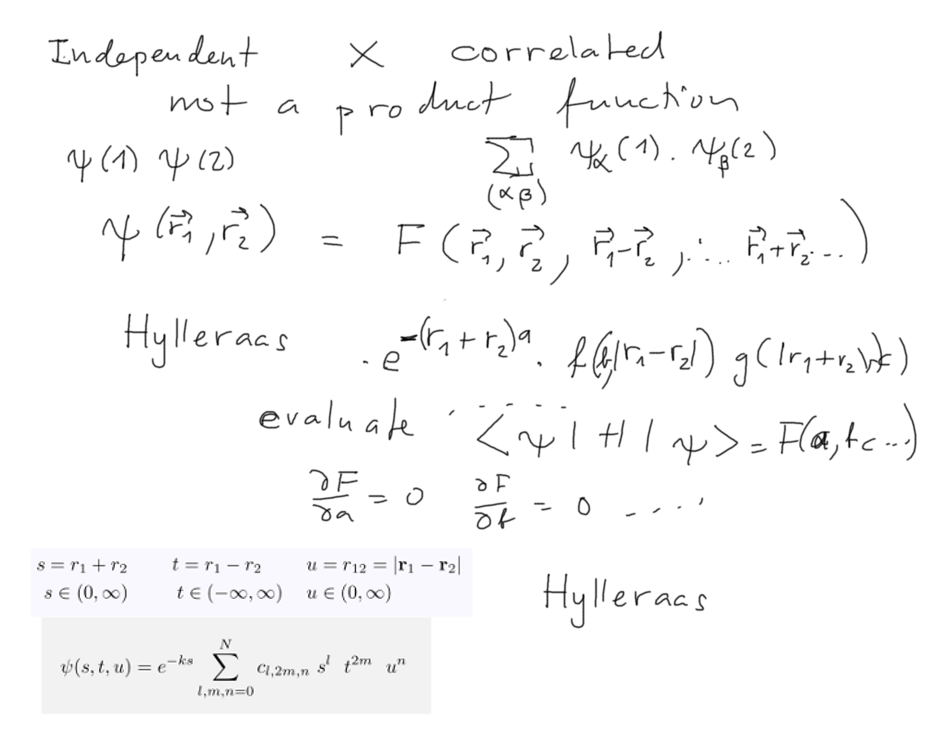
The variational method - best possible effective z - but
INDEPENDENT ELECTRONS
The discrepancy - can be solved by going beyond the independent
electrons.
This is often called ELECTRON CORRELATION - the motion is not
independent - it is correlated
One possible way to include correlation - beyond a product function.
This can be accomplished by a SUM OF PRODUCT FUNCTIONS
(we shall explore this for many-electron systems - configuration mixing)
Alternatively - assume a function of "correlated variables", as e.g.
the distance between the electrons
Hylleraas wavefunction (there are no simple references to be found on
Wikipedia or similar sites.
But Wolfram (Mathematica) has a nice demonstration of the variational
method and the Hylleraas
approach -
http://demonstrations.wolfram.com/VariationalCalculationsOnTheHeliumIsoelectronicSeries/
Unfortunately, their "player" must be installed to experience the
variation. The text is interesting
even without the player)
small_0060.png
small_0060.png
The "correlation variables" used by Hylleraas are in the above inset.
LECTURE NOTE 2013.09.12
to index
2013.09.10 -
previous lecture note
2013.09.17 - next lecture
note











