Many
Electron
atoms
- part 2
SCF-potentials and Total energy of N-electrons in a Slater
Determinant
Explaining the periodic table
- SCF-potentials
In this we are trying to explain why the potentials which
are not purely Coulomb - or Kepler - lead to different
energies for the same n but
different orbital momentum quantum number l through the "centrifugal
potential" addition
(l (l+1)) / r2
We modelled the SCF potential by its limiting behaviour - close to the
nucleus - Coulomb for charge number Z
far away - Coulomb for charge number 1 - the last electron does not see
the whole charge, that is screened away
by the (Z-1) others.
So why are the energies different? The pictures show it.
a010.png

a010.png
Periodic table is thus explained by the centrifugal barrier.
Because of that the s-states of n+1
- shell come below the d-states of the n - shell
At the end we mention what else can be studied BEYOND the independent
electrons and the SCF
TERMS - splitting of configurations, rest-interactions after the SCF is
determined. This and configuration
mixing
will be discuessed later
Total energy of N-electrons in a Slater Determinant
Now we turn to a more formal work with the SCF. Instead of the
qualitative picture of the
AVERAGE POTENTIAL above, we start with Schrödinger equation
for N electrons
First N=2, already mainly done. Consider only the antisymmetric
spatial function
(for (n,l)(n',l') configuration ) 2-terms in each wavefunction, 3 terms
in the hamiltonian,
thus 12 terms. We shall see that many will be zero
and then many will be identical.
We shall arrive at two statements:
1. for single-particle terms -
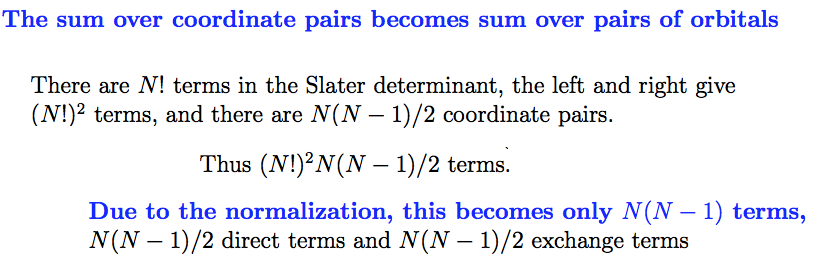
The sum over
coordinates turns into a sum over orbitals - with no additional
N-factors (or n as used in the note)
The n!-terms in
the normalizationcancel with the number of n! various terms - which are
identical
2. for pair interaction:
The sum over
pairs of coordinates turns into a sum over the pairs of orbitals
- but with a doubling
due to the
DIRECT and EXCHANGE terms
We start with the normalization - and go straight to the single
particle terms
a020.png

a020.png
Here we state why the terms become zero. The single particle
operators can have terms of type <a|O|b> wit a
different from b,
but then there will be <b|a> which gives the zero
a030.png

a030.png
Thus this explains the first "theorem" here at least for 2 electrons,
but as well for N-electrons:
1. for single-particle terms -
The sum over
coordinates turns into a sum over orbitals - with no additional
N-factors (or n as used in the note)
The n!-terms in
the normalizationcancel with the number of n! various terms - which are
identical
Now we go over to the PAIR term in case of n=2, helium
a040.png

a040.png
In the helium case we see the origin of the direct and exchange term.
But the sum over pairs - it is ONLY one pair.
But we see why the normalization cancels with the number of IDENTICAL
REPETITIONS - in this case TWO.
And we start on the Lithium N=3 case - continued below
Here is the helium revisited and summarized.
b01.png

b01.png
b02.png
b02.png
Here we show why again most of the terms will be zero
<a b | D | c d> would be nonzero, but would be killed
by < c d | a b > being < c | a >
< d | b > - thus zero times zero.
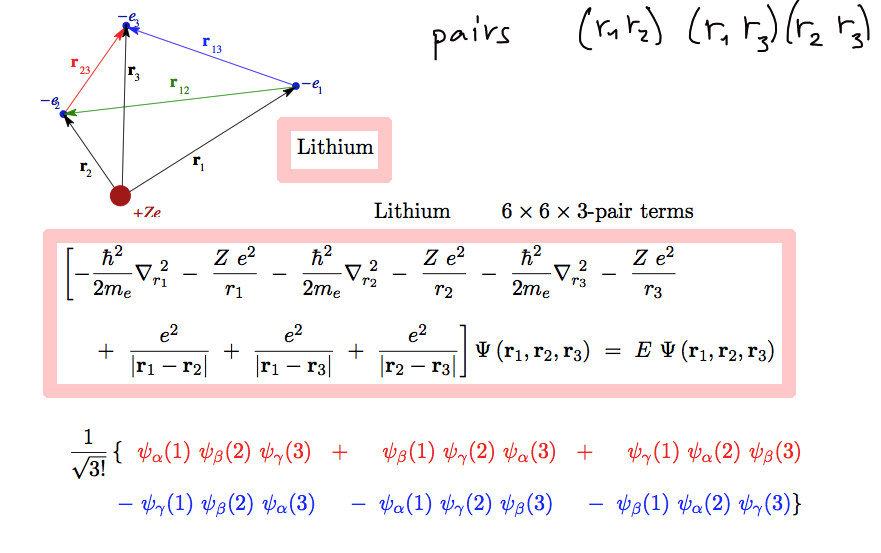
Below it is illustrated for the Lithium case
b03.png
b03.png
Lithium case - 3! is 6 ( n! )(n!) is 36. But as
shown only 12 of these will be nonzero - and six of them will be direct
6 of them
will be direct.
And then it will be repeated for all coordinate pairs - and the number
will simply fit
so that this demonstrates the second "theorem"
2. for pair interaction:
The sum over
pairs of coordinates turns into a sum over the pairs of orbitals
- but with a doubling
due to the
DIRECT and EXCHANGE terms
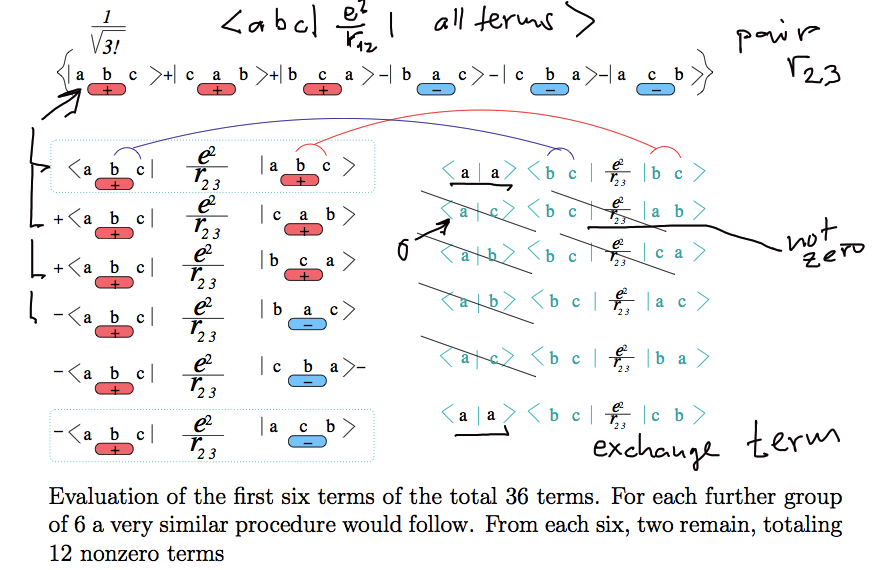
b04.png
b04.png
... from the text
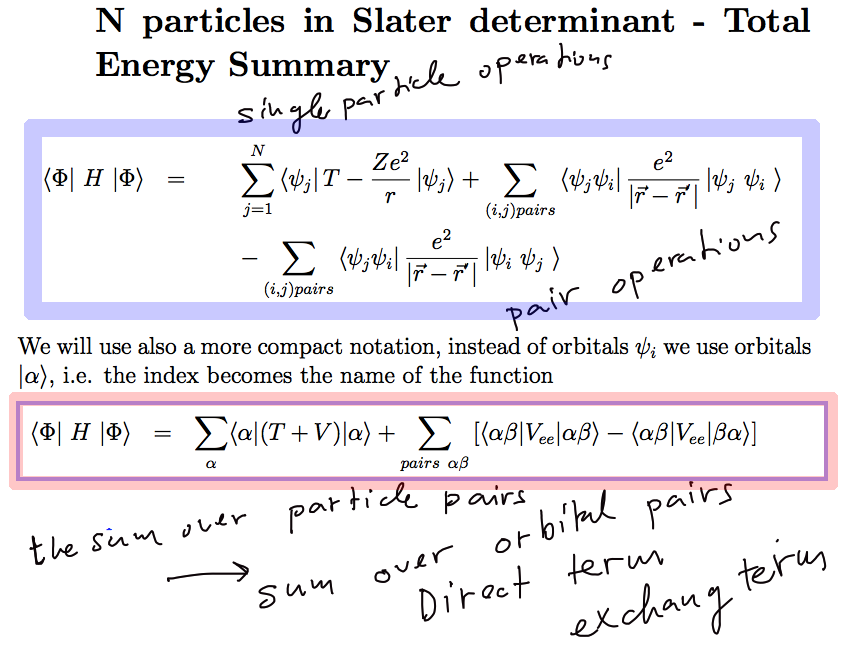
b05.png
b05.png
Thus we have really shown that
1. for single-particle terms -
The sum over
coordinates turns into a sum over orbitals - with no additional
N-factors (or n as used in the note)
The n!-terms in
the normalizationcancel with the number of n! various terms - which are
identical
2. for pair interaction:
The sum over
pairs of coordinates turns into a sum over the pairs of orbitals
- but with a doubling
due to the
DIRECT and EXCHANGE terms








