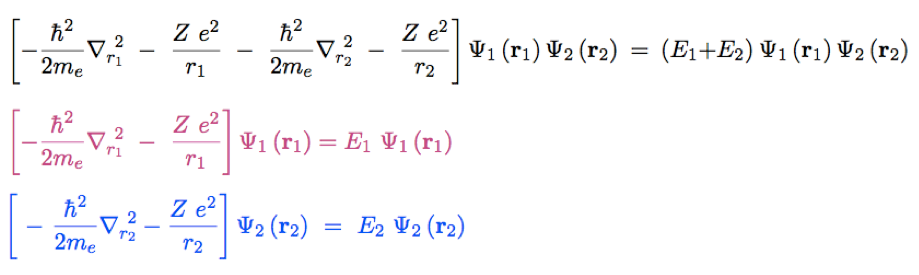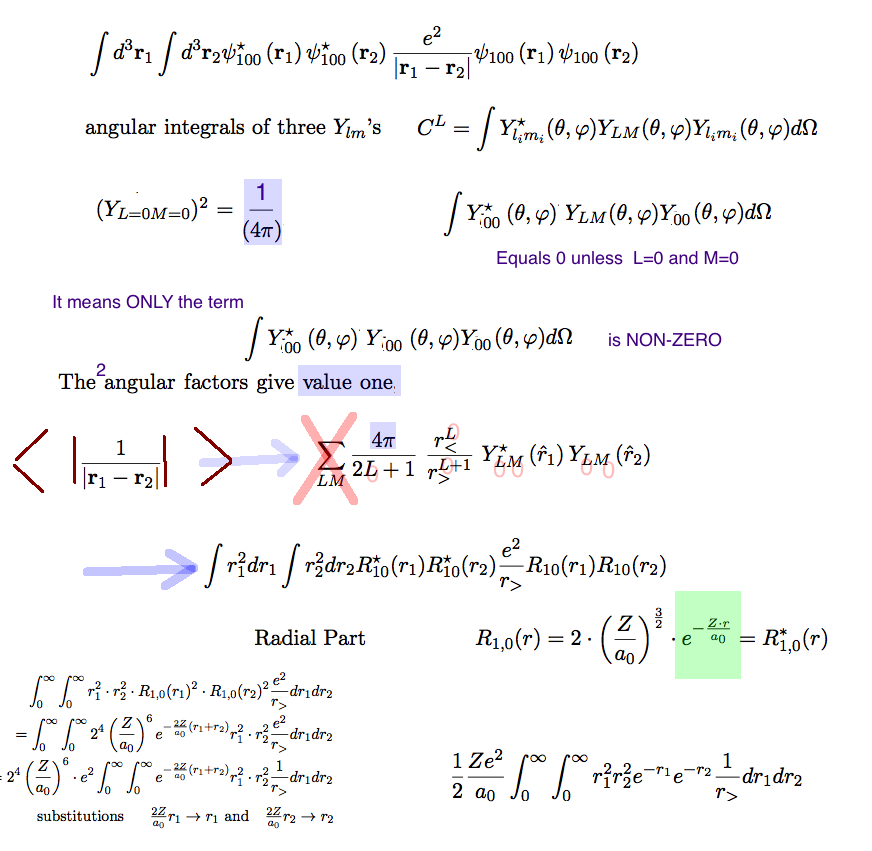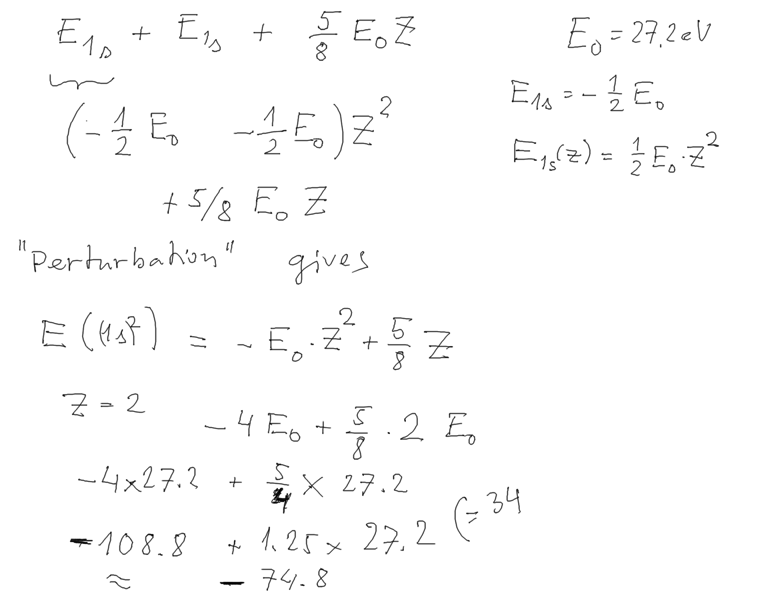Energy of the ground state of 2-electron atoms -
electron repulsion
Lecture 01.09.2011
Links from last time for Hydrogen-like wavefunctions:
Hydrogen Atom
Independent electron
approximations
(product wavefunctions, separation of electron equations,
perturbation theory .... )
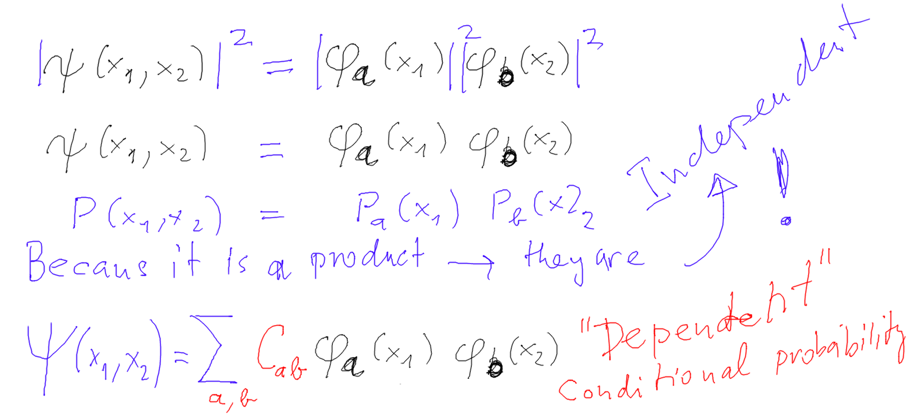
We start discussing the expression "independent particles" when
using a product function.
Product function describes "independent particles" because the
PROBABILITIES are for
independent events ....
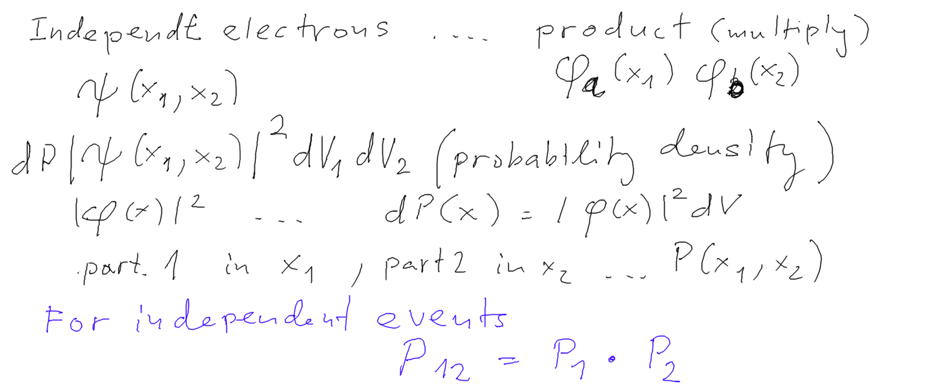
Elementary probability theory: occurence of independent
events - the probabilities are multiplied
Our probability densities are products -> independent particle
events -> independent particles
When particles are not independent ....
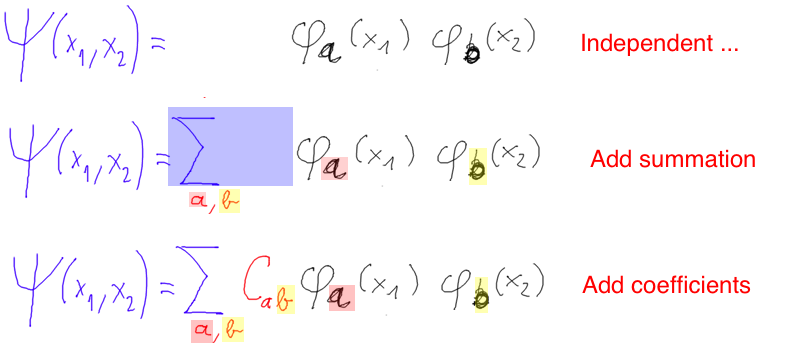
from-independent-to-entangled-particles.png
Using the independent electron (product wavefunction)
Evaluate expectation value of the total energy

3-expectation-value.png
Using the independent
electron (product wavefunction)
Evaluate expectation value of the total energy
THIS EVALUATION - we lost
some of the note (crashed the the painting program) Here the
saved version is edited to this:
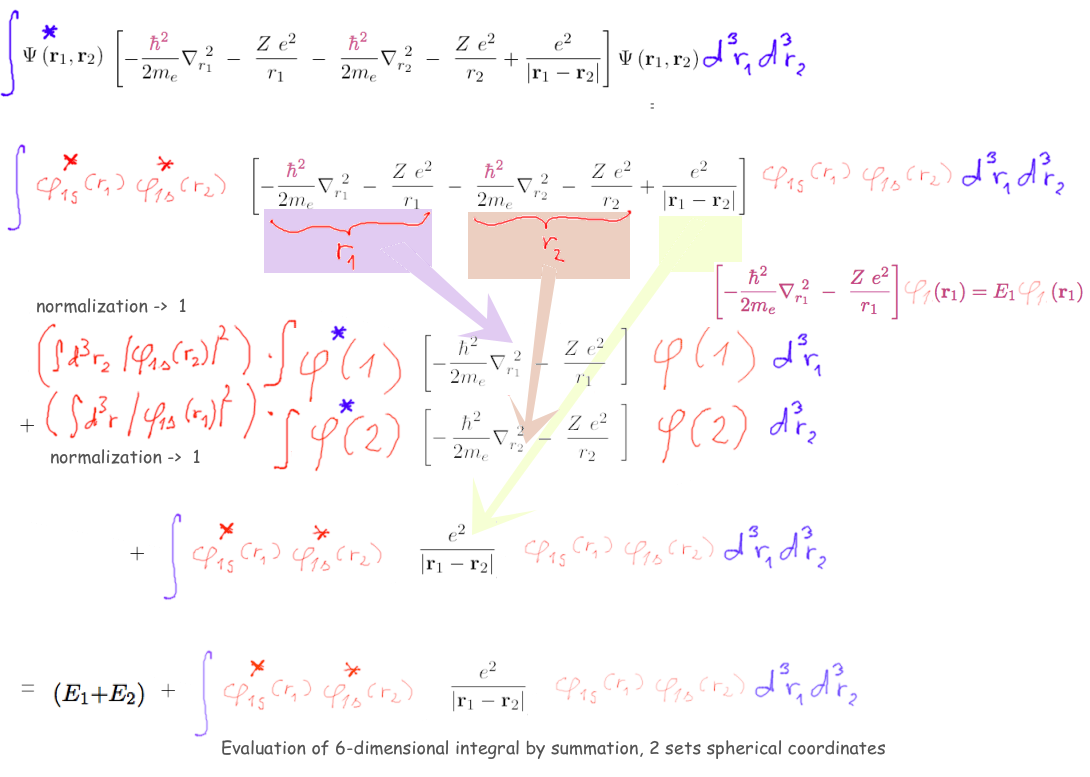
The first 4 terms are easily evaluated above - leading to E1
and E2
what remains is one 6-dimensional integral of the REPULSION
This has been discussed last time already
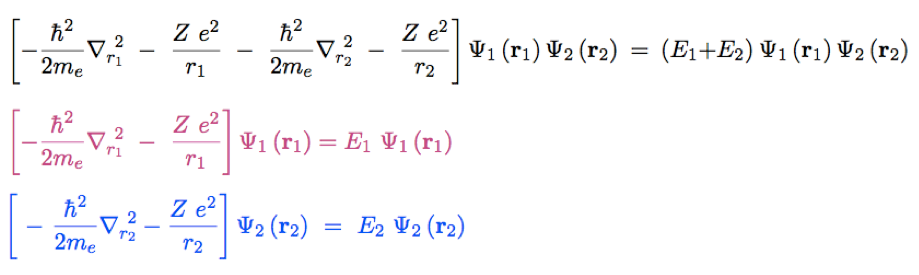
- we us this now
The evaluation of the repulsion term has been shown following
the PDF NOTE
I have redone by taking snapshots, re-arranging them, added
annotations - arrows, etc ...
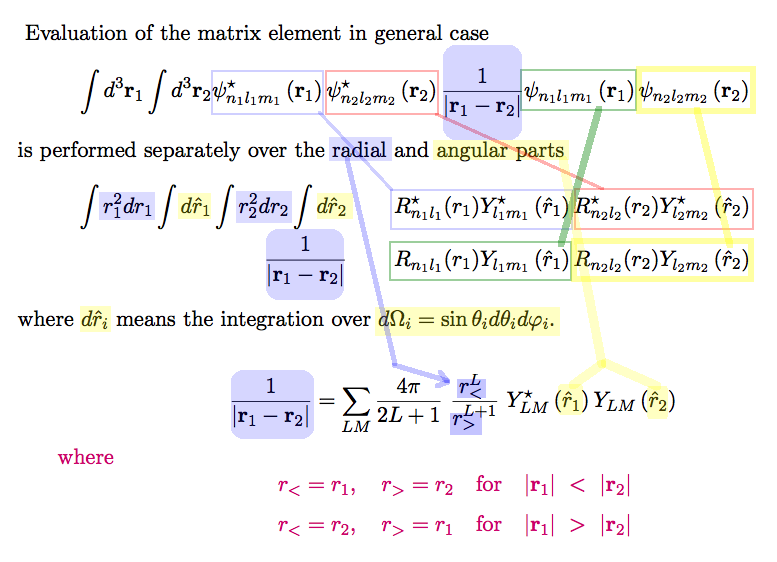
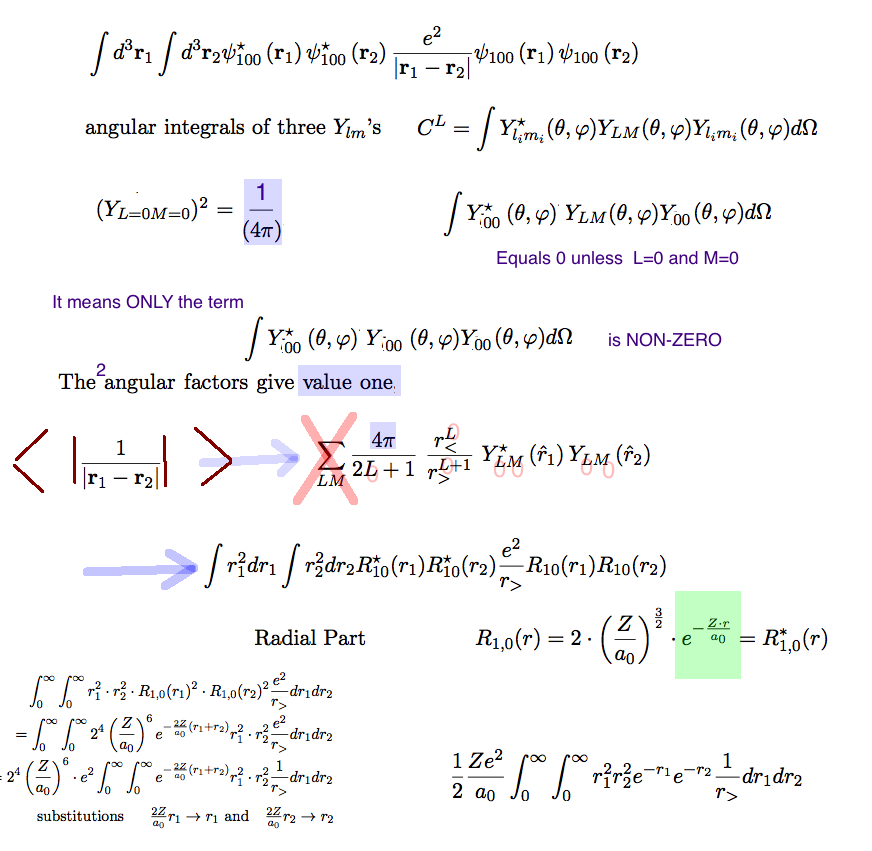
HERE the 6-dimensional integral has been reduced to a double
integral only
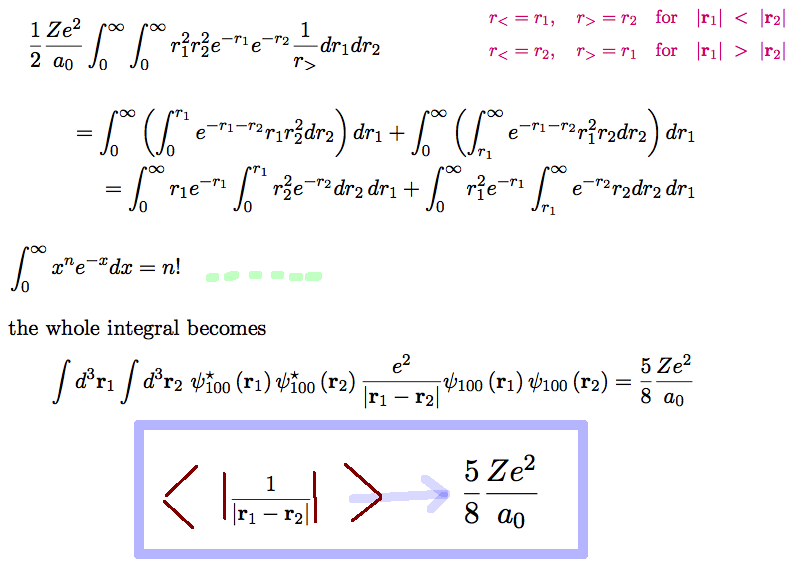
evaluate-repulsion-result: for all Z
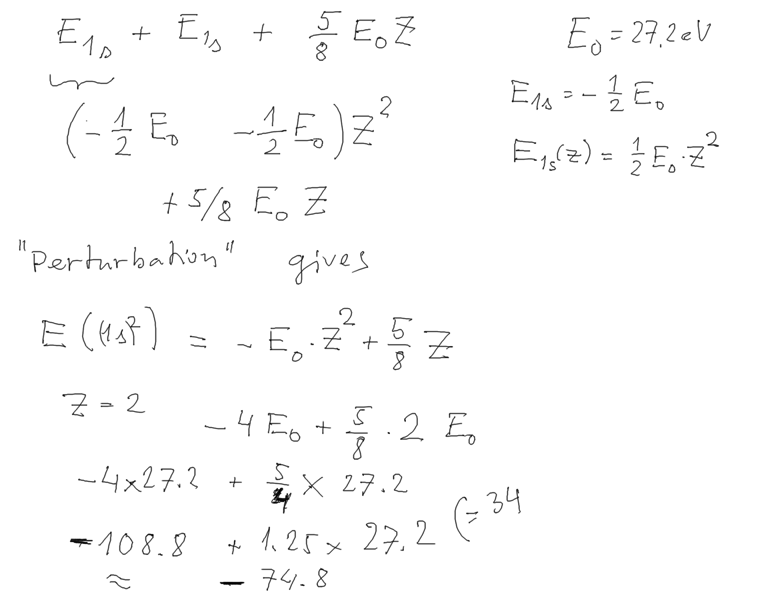
result-perturbation-expectation.png
Perturbation theory result
for all 2-electron atoms, starting with negative hydrogen
ion
we concentrate now on the He case, evaluated above
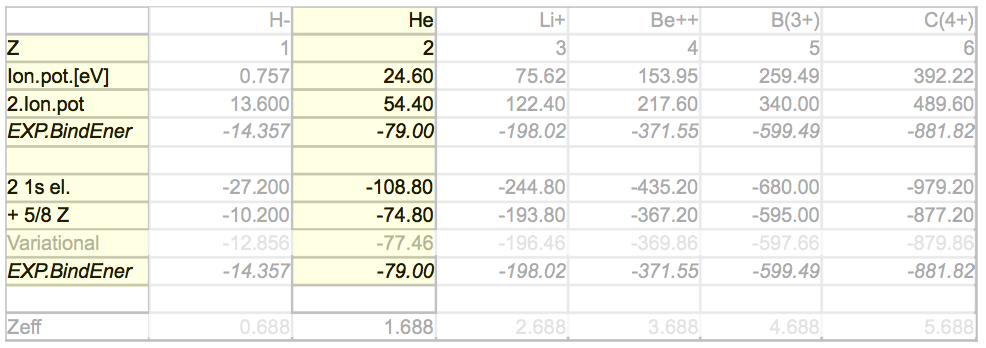
result-perturbation-TABLE
Spectra of He I He II
neutral He, ionized He - and
the notation He I, He II
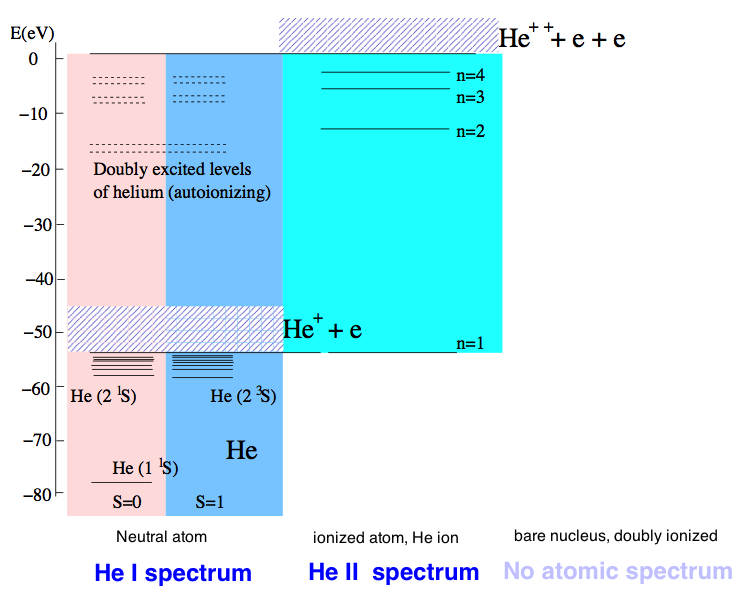
We have visited the Database at
Note the units of energy used (hint: wavelengths.pdf )
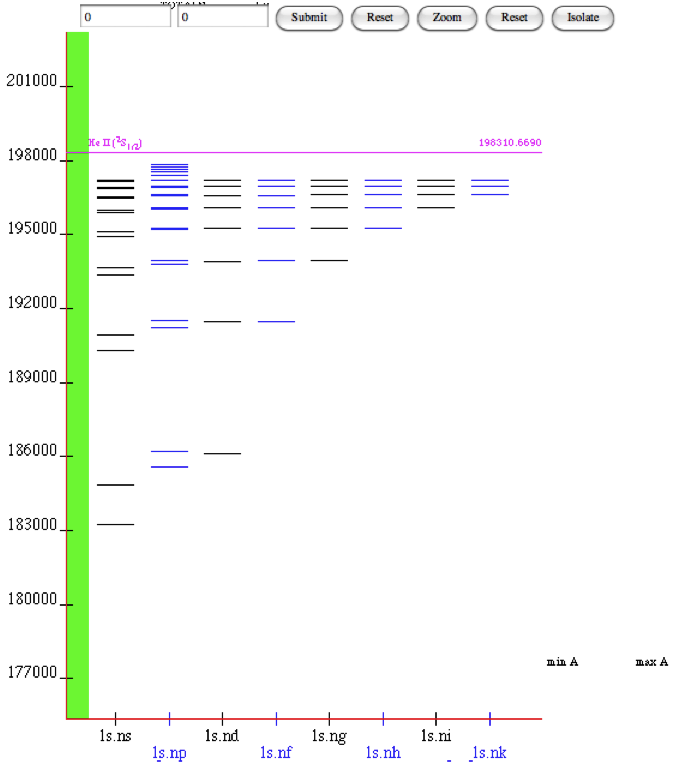
He-I-atNIST-database
other links:
Our research
http://iopscience.iop.org/0953-4075/labtalk-article/47053
Next Time:
1. Improvements in the independent electron model - VARIATIONAL
METHOD
2. Electron spin - symmetry and antisymmetry, excited states of
2-electron atoms