Helium - From Pauli exclusion to Configuration mixing
From Pauli exclusion to Symmetry principles
Identical particles came from statistical ideas,
symmetry - or antisymmetry gives the exclusion behaviour

1-Pauli_principle_to_Exchange_Symmetry.png
Spin and space motion must be independent - there is no term
which depends
both on space and spin coordinates (spin is totally absent in our
total energy ...
THUS - PRODUCT FUNCTION
and each must be "symmetrized independently

2-spin_symmetry_and_space_symmetry.png
Two spins - we look at the z-component (see last lecture
2011.09.06 previous lecture
note )

3-spin-symmetric-antisymmetric.png
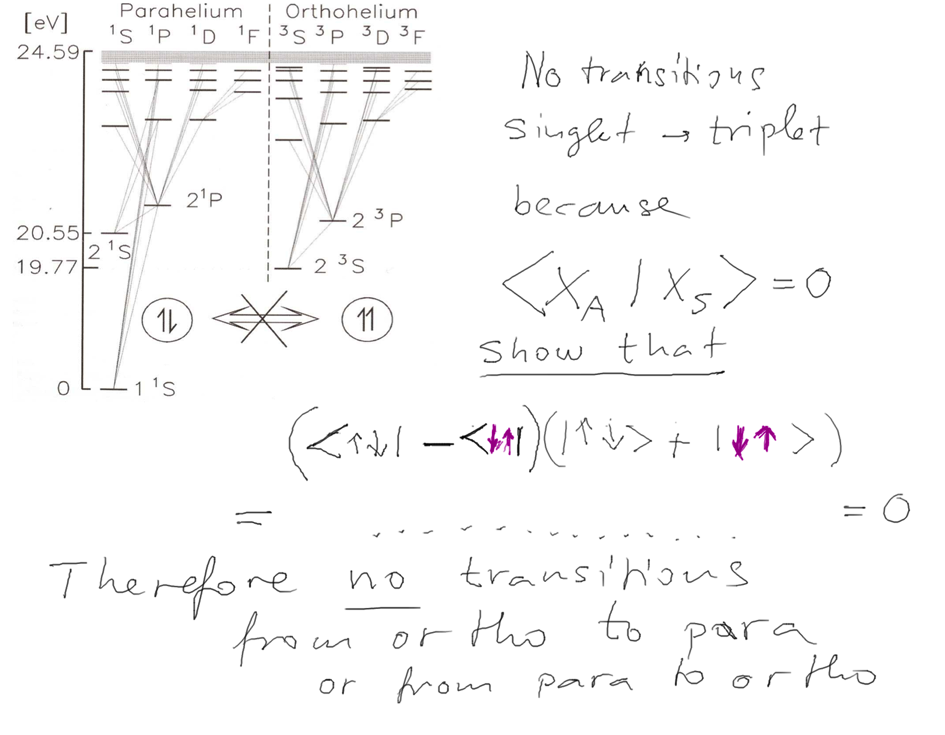
Now we look at the ORTHO - PARA difference - in repulsion

4-symmetry_repulsion_in_ORTHO_and_PARA.png
Why ortho (the "right one" for triplets and para,
the "unusual, paranormal, exotic, not real" - for the singlets?
Ground state is a singlet!)
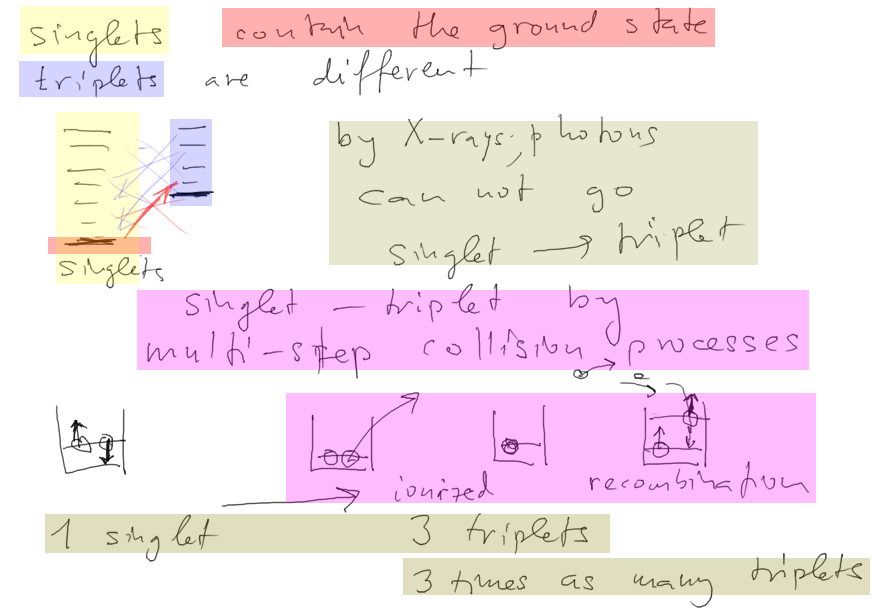
probable reason:
in collision excitation (heating etc) triplets can be generated 3
times more often,
simple because of randomness
5-ortho_para_creation.png
5a-ortho-para-transition-not-possible.png
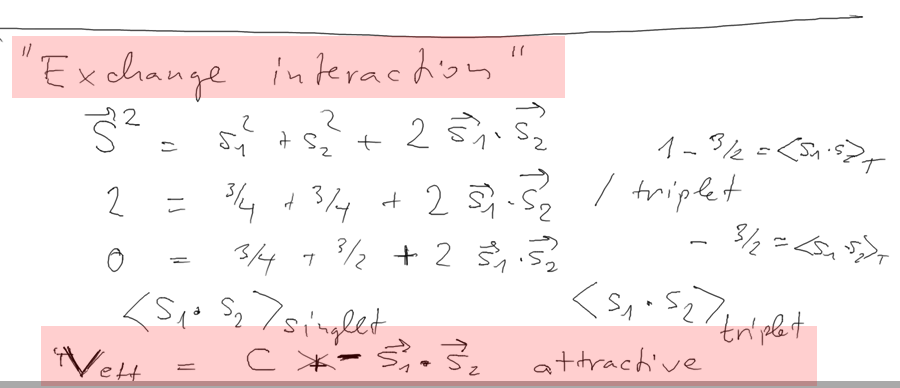
About the effective exchange interaction - and
ferromagnetism
6_effective_exchange_interaction.png
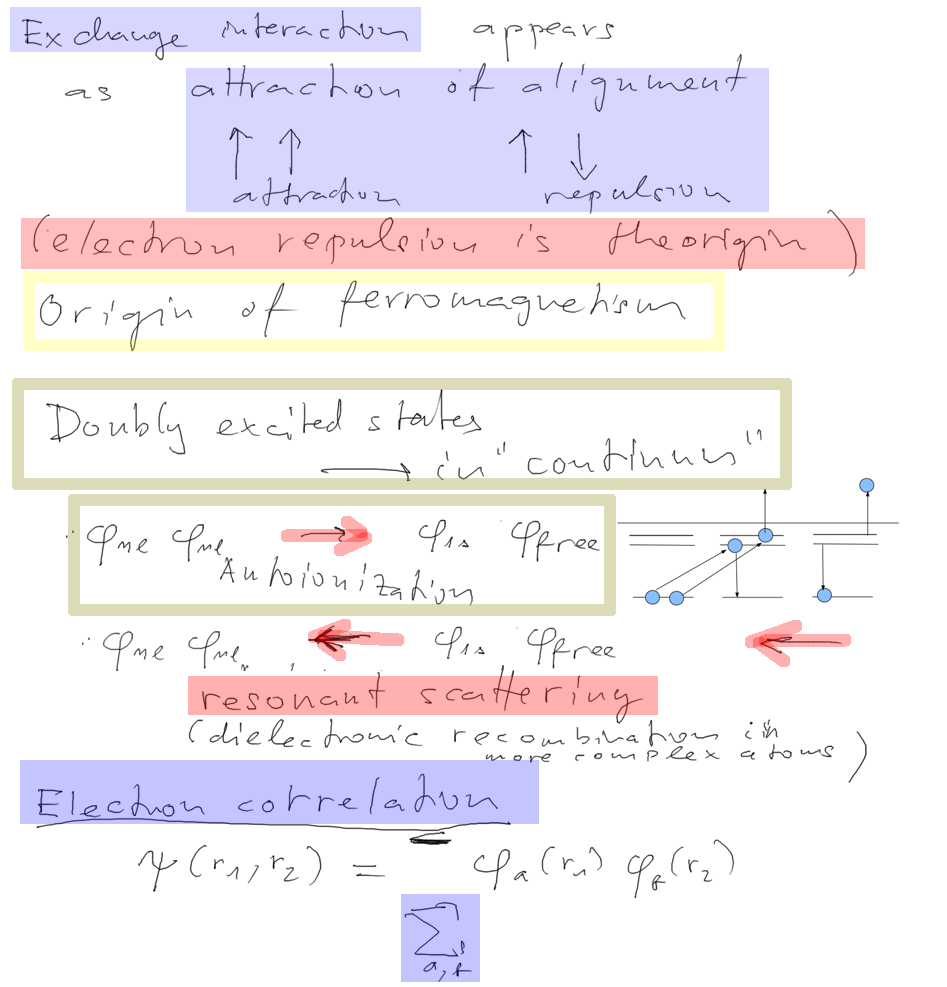
Here we discussed exchange interaction and
ferromagnetism
but continued to Doubly excited states
At the end of this slide we started on the following -
Electron correlations
7-exchange_ferromagnet_Double_excited.png
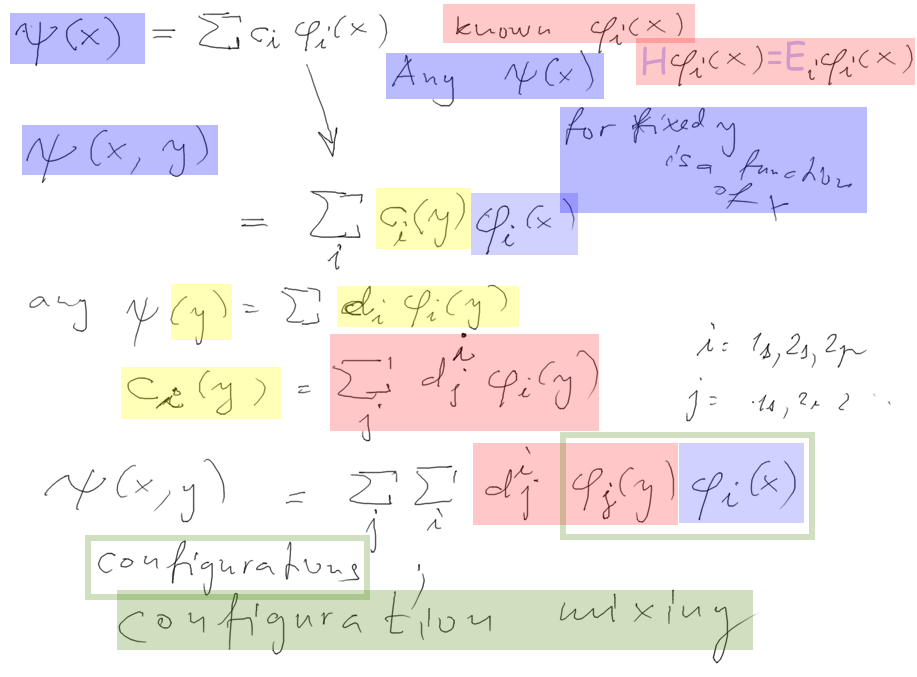
Electron correlations - and configuration
mixing
Electrons correlated - not
independent. THUS NOT PRODUCT FUNCTION
It can not be f(x)g(y) product, it must be general F(x,y) type
But then we can do the expansions .....
8-configuration-mixing.png
Next time
Concluding the Helium
Starting Many electron atoms
Preparing to start on Optics part








