Discussed also:
Light_spectrum.svg (copied from
wikipedia )
Two-electron atoms; Slater determinant for 2 particles
Here we show the evaluation of EXPECTATION VALUE of energy
we obtain the exchange interaction in formal way
We shall repeat this once more as special case of "many electron atom"
with many=two

_00_slater_determinant_Helium.png
Hylleraas variational
function (made by hylleraas.tex.txt
)
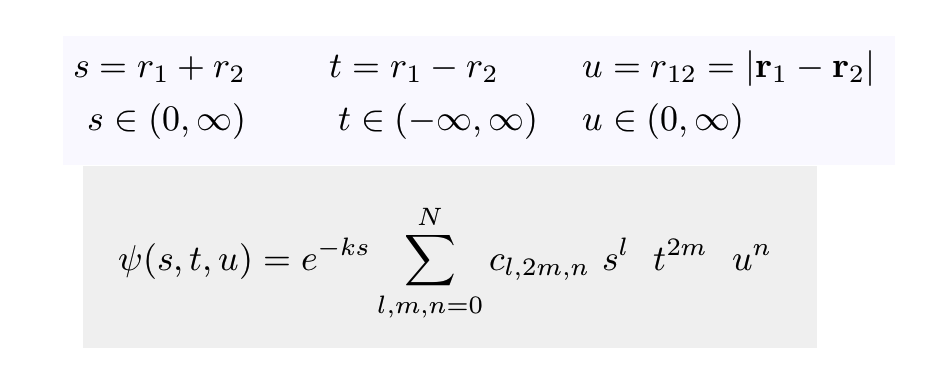
__0_Hylleraas_variational_function.png
Variational method: the parameters are k in the exponential and
all the unknown coefficients cl,2m,n
Variational method - conditions for minimum give necessary number of
simultaneous equations.
The system of equations must be solved to get the values of the unknown
parameters
Many electron atoms
aufbau-empty.png
|
aufbau.png |
Binding energies of atoms with changing Z
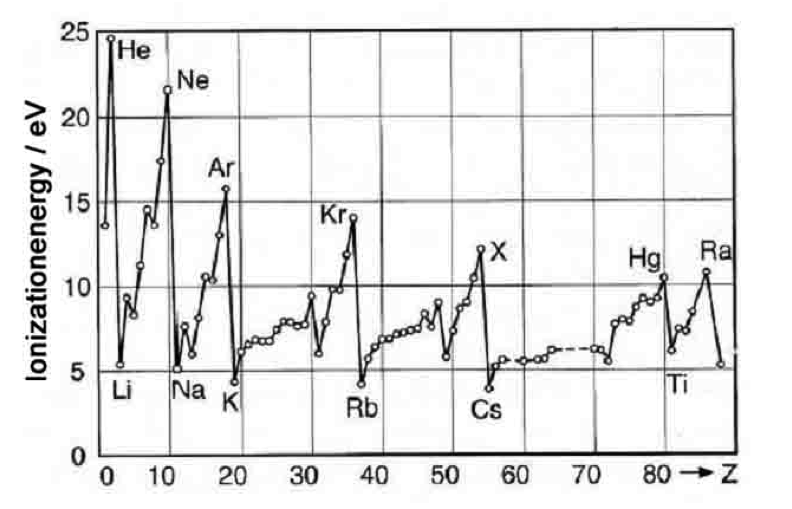
Ionization_energies.png
Model for selfconsistent field
Interaction of 1 electron with a "cloud" of charge - the other
electrons.
First we consider interaction of one electron with a another charge.
Assume density distribution. Charge in one volume element.
Sum - or integrate over the whole space
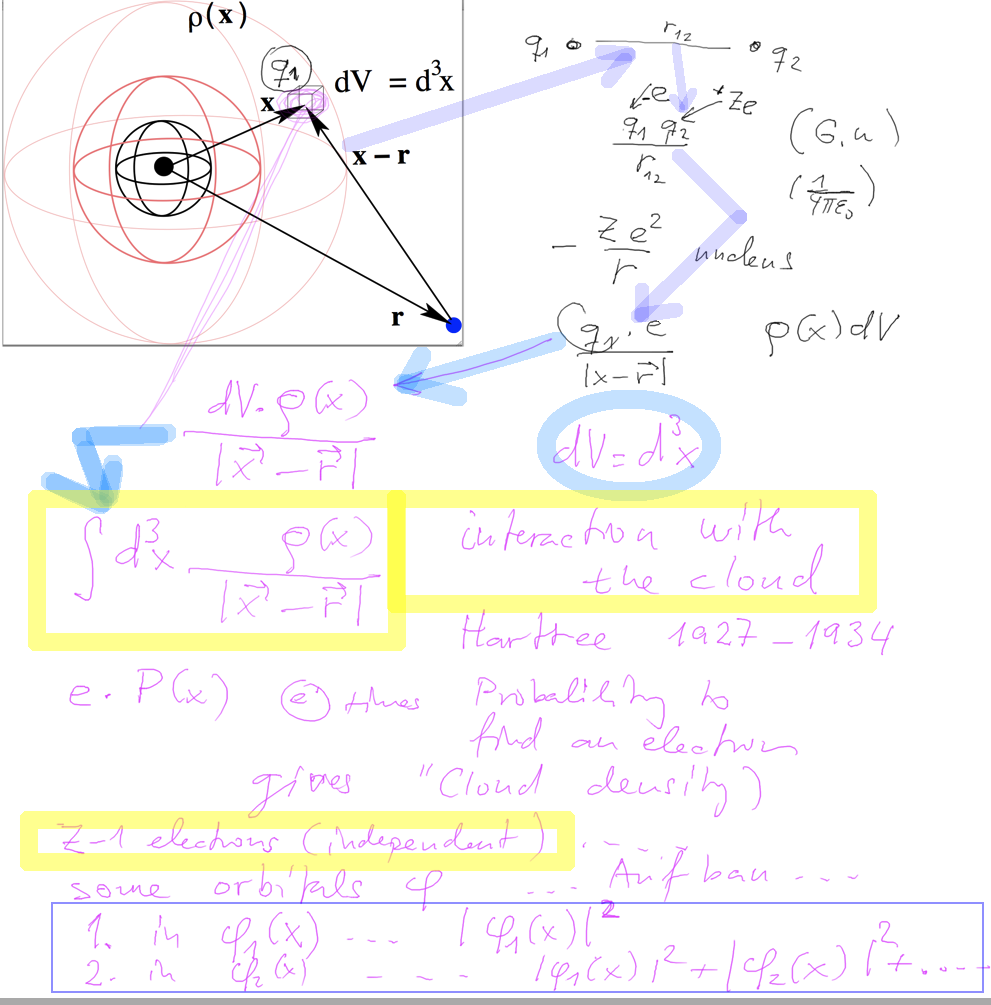
0_electron_electron_cloud.png
Independen Z-1 electrons - probability of meeting any electron - sum
over probability of each of them (not multiply here, sum!)
Constructing the density for arbitrary Z orbitals. Start e.g. with
hydrogen-like orbitals.
(Why Z and not Z-1 It should be Z-1, this is an approximative treatment
- to have one
potential for all orbitals.
Outlined is the SELFCONSISTENT field approach - ITERATION
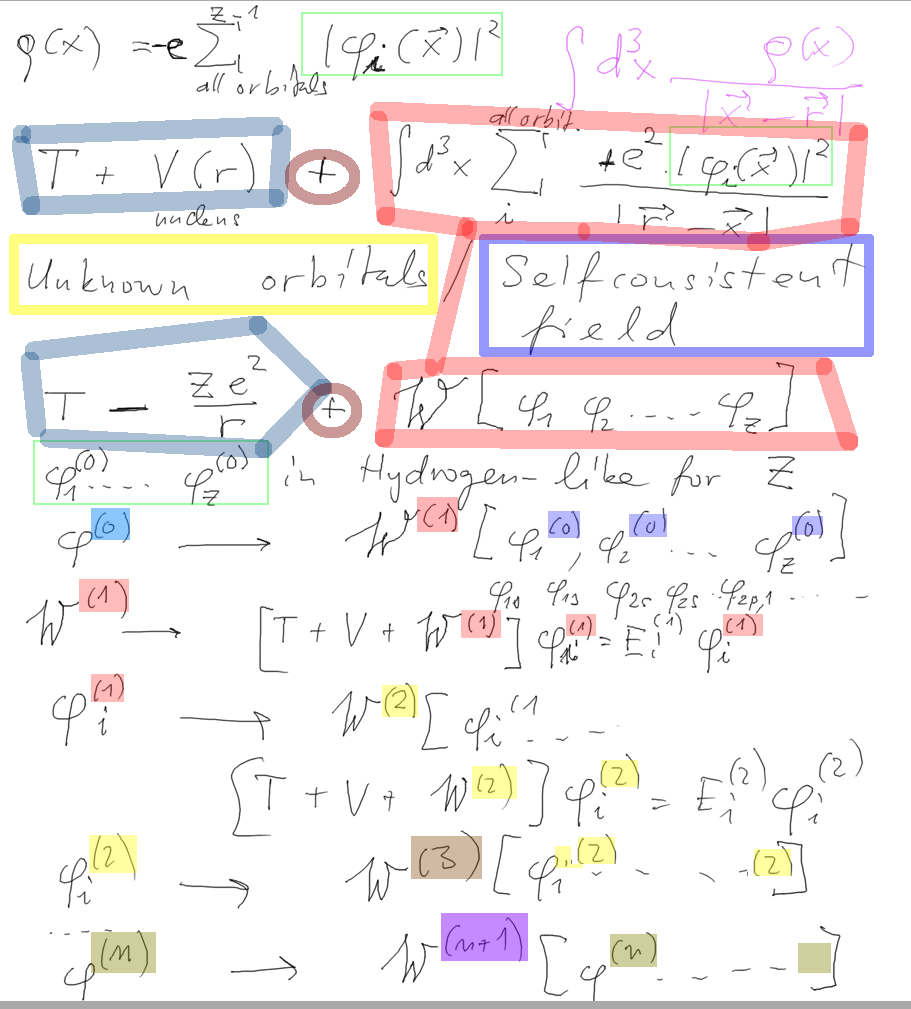 1_selfconsistent_field.png
1_selfconsistent_field.png
Outlined is the SELFCONSISTENT field approach - ITERATION - and
WHEN TO STOP ...
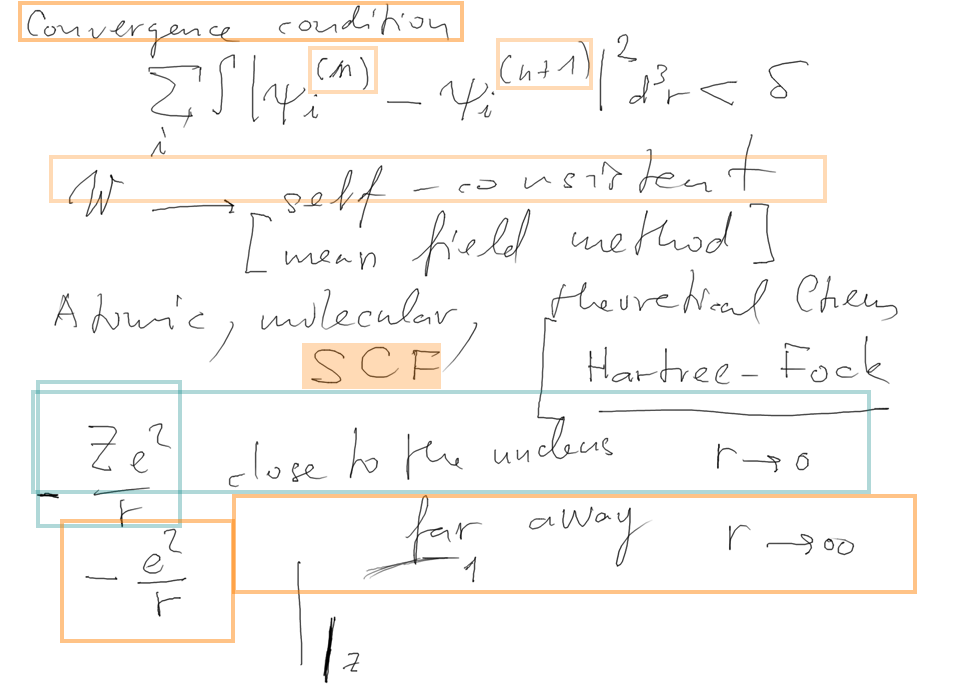
2_selfconsistency_condition.png
SELF CONSISTENT FIELD SCF
used in atomic and molecular physics, theoretical chemistry (quantum
chemistry)
Extra files in this position:
Light_spectrum.svg
hylleraas.tex.txt
2011.09.08 previous lecture
note LECTURE
NOTE
2011.09.13
2011.09.15 next lecture note



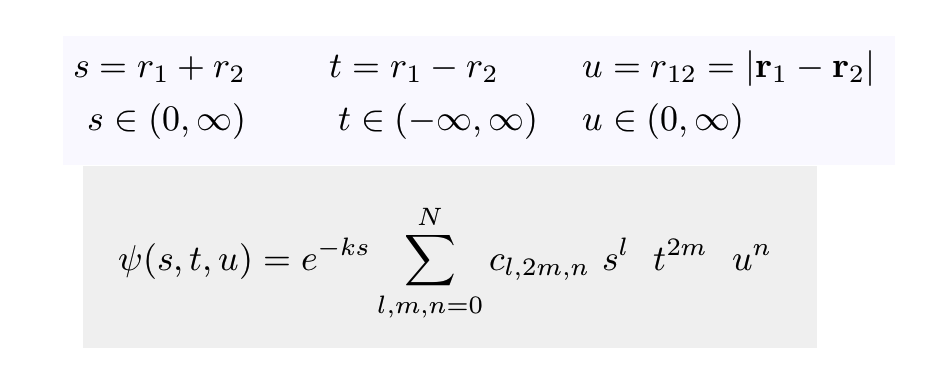




 1_selfconsistent_field.png
1_selfconsistent_field.png 