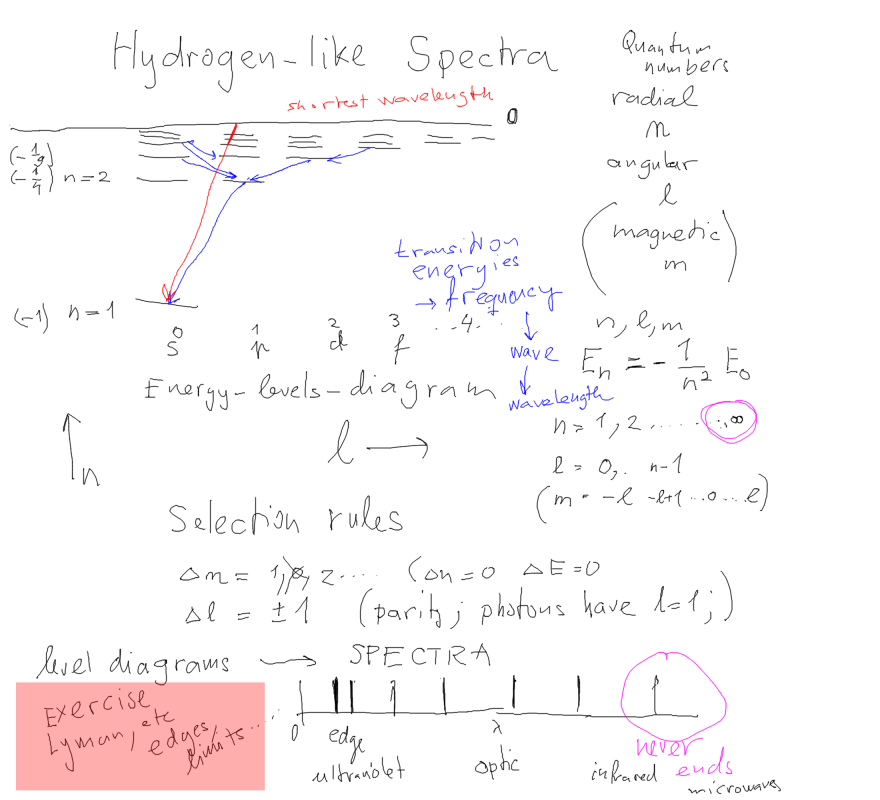
 1-drawing_hydrogen_spectra.png
1-drawing_hydrogen_spectra.png 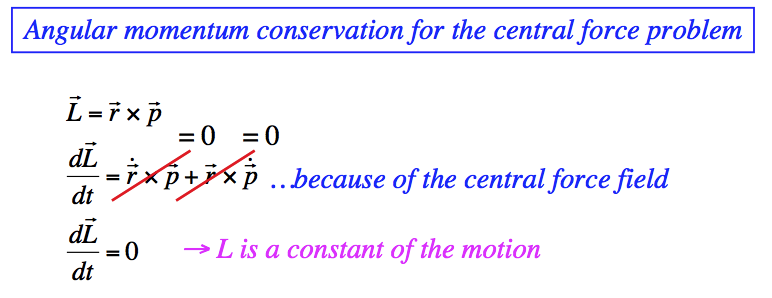 1B-conserve_L_angular_Classical.png
1B-conserve_L_angular_Classical.png 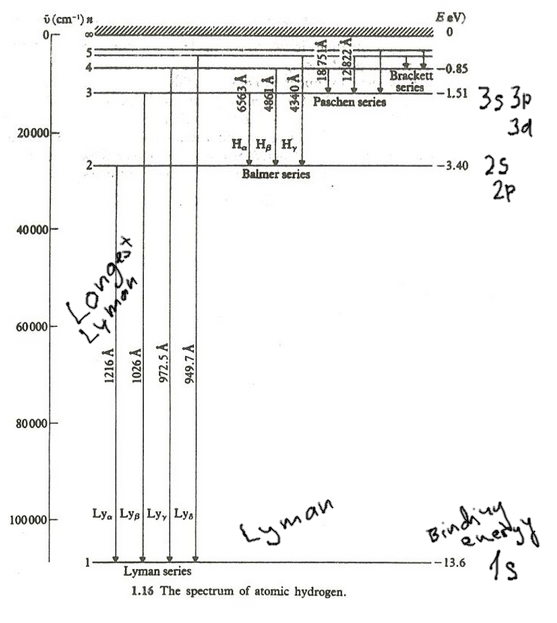 2-Hydrogen-spectrum.png
2-Hydrogen-spectrum.png 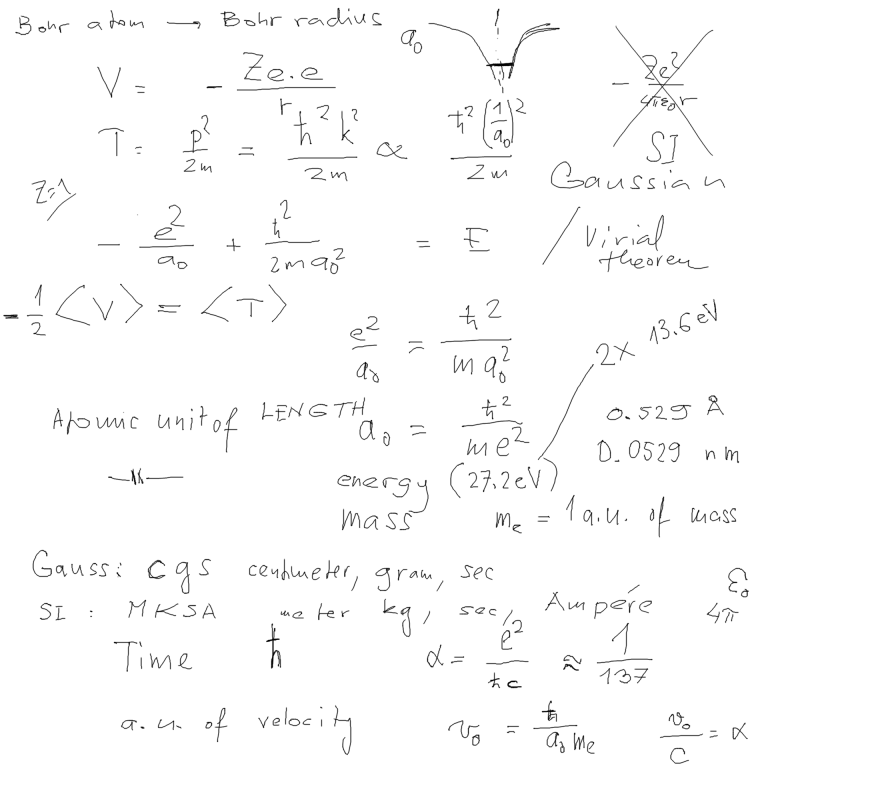 3-atomic-units-derived-defined.png
3-atomic-units-derived-defined.png  4-atomic_units.png
4-atomic_units.png 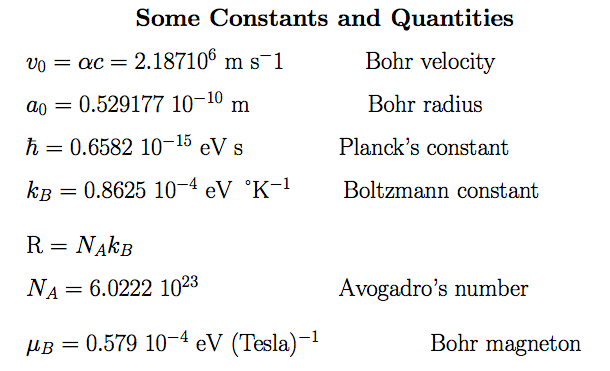 5-some-constants.png
5-some-constants.png Hydrogen - like atoms - CENTRAL POTENTIAL -> Angular momentum conservation - Classical - Quantum Theory Angular momentum --- Y_LM Spherical harmonics Separation of variables - classical, r, theta, phi Separation of variables - Conservation of (all components ) of angular momentum vector (classical) Separation of variables - Conservation of LENGTH of angular momentum vector, or L-squared (QUANTUM) Radial waves general, hydrogen radial eigenfunctions Quantum results - Quantum numbers n, l, m Spectra, Energy level diagrams Atomic Units; Virial Theorem (wikipedia); Johnson's book - Lectures on Atomic Physics |
Spectra History of Atoms - Atomic models (Christmas pudding with raisins) Rutherford; Bohr Schrödinger equation; Wolfgang Pauli Hydrogen Atom wavefunctions Atomic Units; Virial Theorem (wikipedia); Rydberg Atom Orbitals Electromagnetism - Relations, Units Physics formulas are stories in miniature (so is most of math) Closed trajectories, ONLY Kepler and HO, (statistics of the trajectories - QM ) (Circular flat potential well - all closed trajectories ???? NO !!! ) Physics and Computers (world wide web is a child of physics) Fun: Victoria Hart on YouTube (math art) |