Helium atom - and 2-electron ions
First some More on Hydrogen-like atoms - Discussing Spectra; FROM
2012
What are the selection rules, NOTE the "Homework" - construct line
spectrum
(wavelengths) from the "spectrum" as LEVEL DIAGRAM
0001-drawing_hydrogen_spectra_2012.png
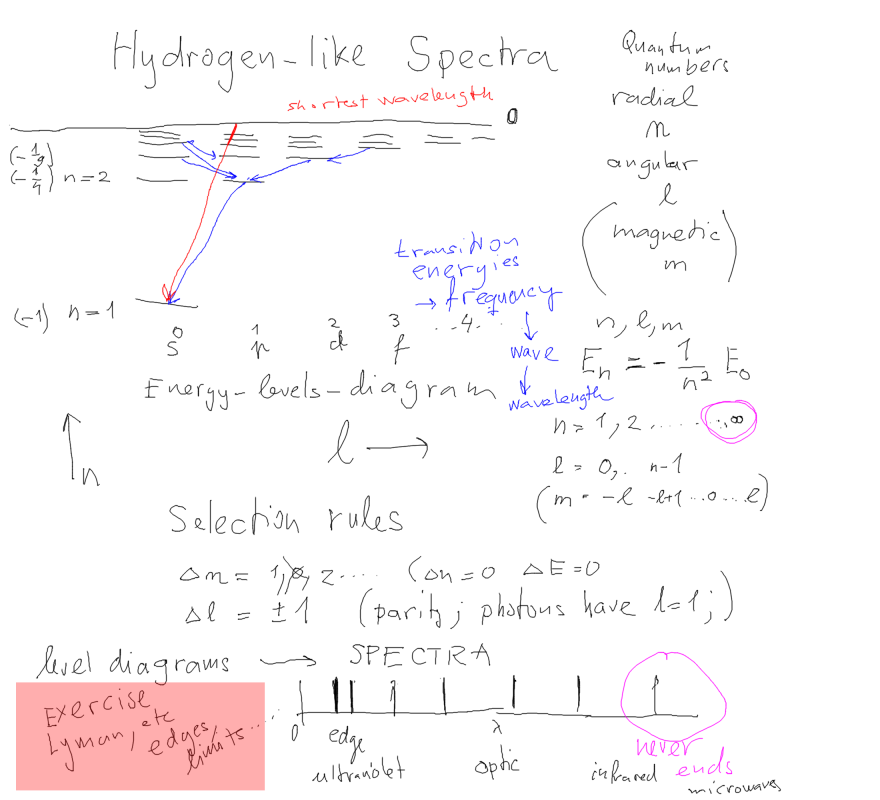
0001-drawing_hydrogen_spectra_2012.png
LEVEL DIAGRAM for hydrogen - and the
transitions - Discussing Spectra; FROM 2012
0002-Hydrogen-spectrum_2012.png
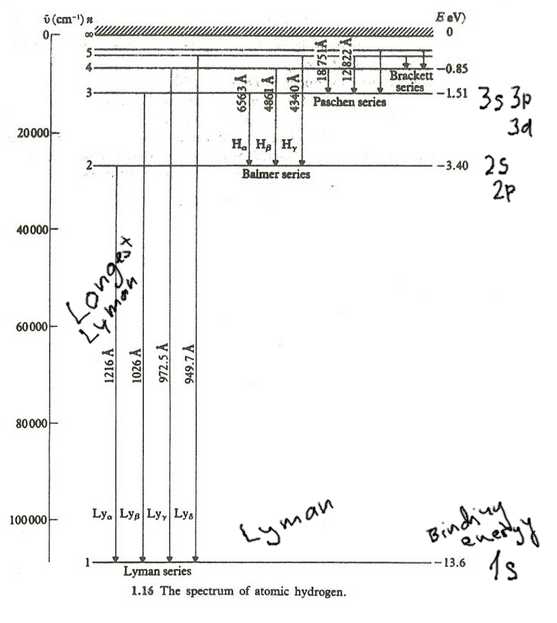
0002-Hydrogen-spectrum_2012.png
Helium atom - and 2-electron ions
The system of charged nucleus and
2 electrons - vs the hydrogenic 2 particle system
Classical mechanics - three bodies make a possibly "chaotic system",
i.e. system
extremely sensitive to initial conditions. ( Sci_fi Novel http://en.wikipedia.org/wiki/Rendezvous_with_Rama
)
Quantum - total energy; 6 coordinates - 2 times x,y,z - Hyperspherical
coordinates?
01a_helium-model-forces-coordinates.png
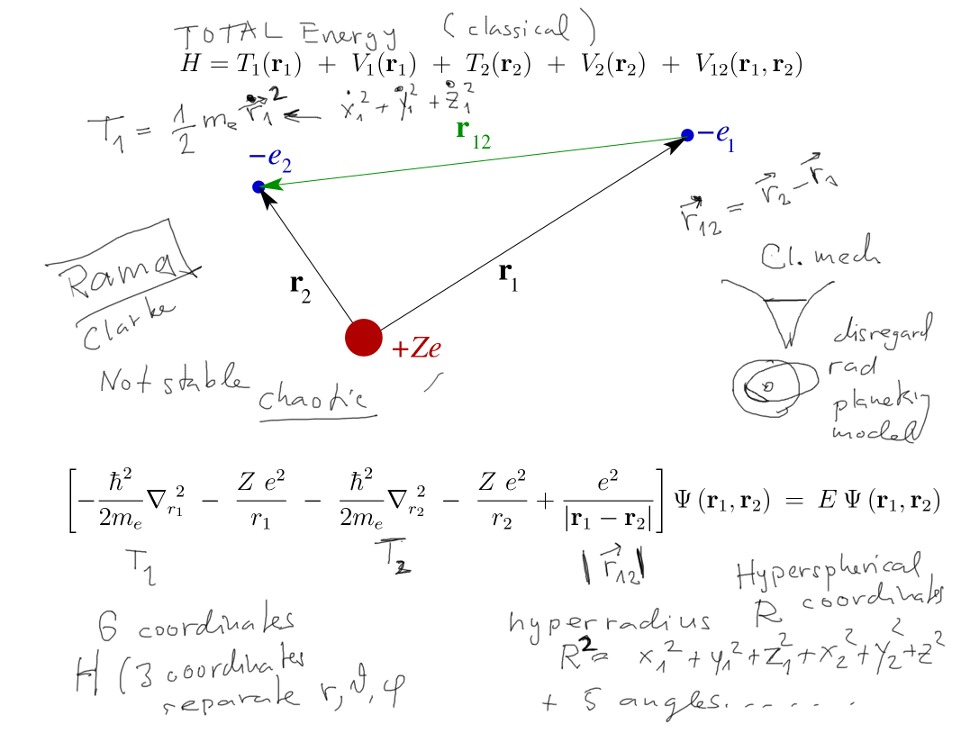 01a_helium-model-forces-coordinates.png
01a_helium-model-forces-coordinates.png
Hyperspherical coordinates http://en.wikipedia.org/wiki/N-sphere#Spherical_coordinates
The concept of independent particles - the product wavefunctions
- How to do Schrödinger for more than 1 particle?
02-independent-particles-separation-neglecting-repulsion.png

02-independent-particles-separation-neglecting-repulsion.png
When interaction neglected - independent particles follow - the product
wavefunctions
The same written on the lecture note independent-
particles separation neglecting repulsion
02-more_on_independent-particles-separation-neglecting-repulsion.png

02-more_on_independent-particles-separation-neglecting-repulsion.png
From independen particles further - Superposition of
"configurations"
( this will later
become configuration mixing )
So the "states" are to large degree products? Experiment, terms, Ground
state mainly 1s-1s or ( 1s ) 2
Pauli exclusion principle postulated in early 1920s
Later justification from "indistinguishable" or "identical" particles
Product wavefunctions essential in understanding
03_Pauli_principle_constructed_from_symmetry.png

03_Pauli_principle_constructed_from_symmetry.png
Exchanging states - or exchanging coordinate
(particles)
Pauli exclusion principle postulated in early 1920s
Later justification from "indistinguishable" or "identical" particles
Probability Pab(1,2)
= Pba(1,2)
= Pab(2,1)
( Meaning Pab( r1, r2) = Pba( r1, r2)
= Pab( r2,
r1 ) )
(one electron in orbital a,
in point r1, and one electron in orbital b, in point r2
)
Product wavefunctions essential in understanding
Exchanging states - or exchanging coordinate
(particles)
04_swapping_of_particles_inside_probability_density_exchange_symmetry.png

04_swapping_of_particles_inside_probability_density_exchange_symmetry.png
Pauli exclusion principle postulated in early 1920s
Later justification from "indistinguishable" or "identical" particles
Product wavefunctions essential in understanding
Exchanging states - or exchanging coordinate
(particles)
Exchange Symmetry - Group theory - Permutations
05_Pauli_principle_based_on_Fermion_exchange_symmetry.png

05_Pauli_principle_based_on_Fermion_exchange_symmetry.png
Slater determinant - for the two electrons Exchanging
states - or exchanging coordinate (particles)
i.e. exchanging orbitals - or exchanging coordinate
(particles) - rows and columns in the determinant


 01a_helium-model-forces-coordinates.png
01a_helium-model-forces-coordinates.png




