Many Electron Atoms - part 1
Pauli Principle; Self-consistent field
Pauli principle again; Number of
oritals for n-th shell
Building-up Atoms
xcf_0010_Pauli_Number_orbitals_Ionization_Energies.png
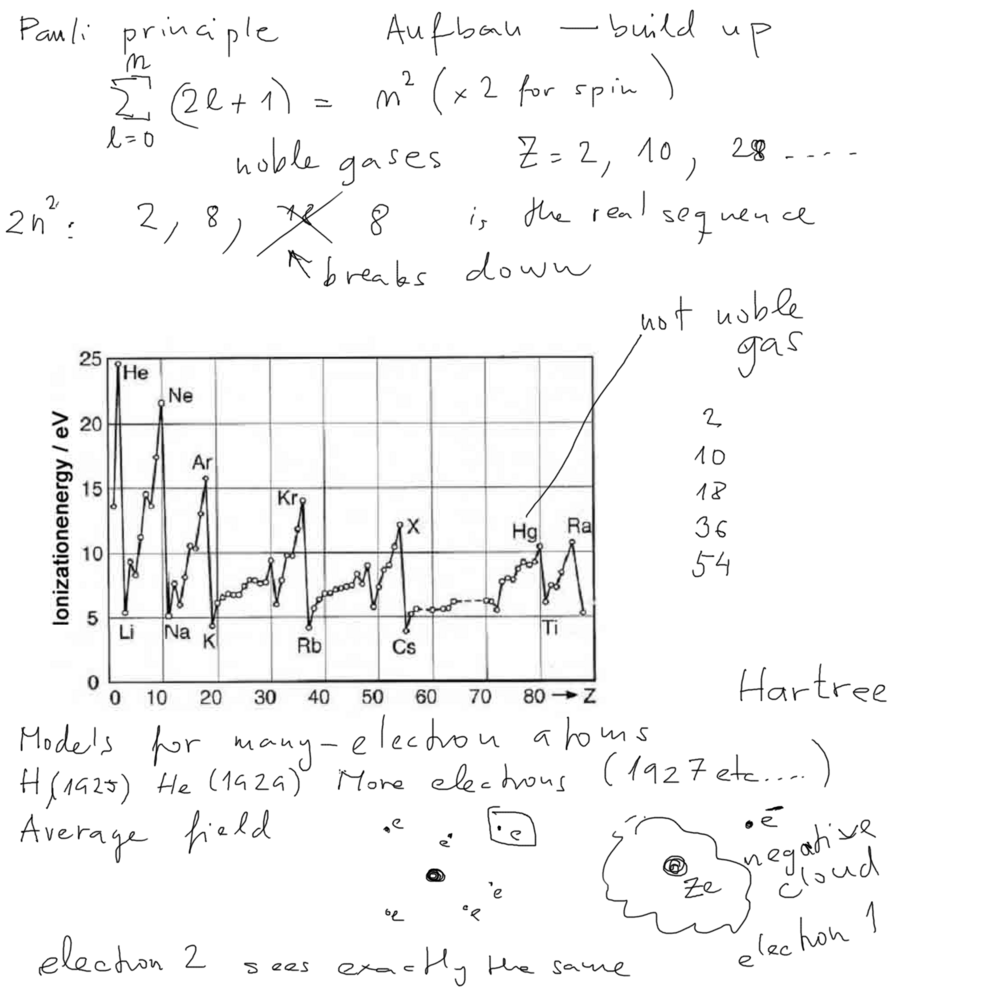
xcf_0010_Pauli_Number_orbitals_Ionization_Energies.png
Building-up
Atoms 1s 2s 2p
3s 3p
3d 4s
4p 4d
4f 5s
Aufbau__building-up.png
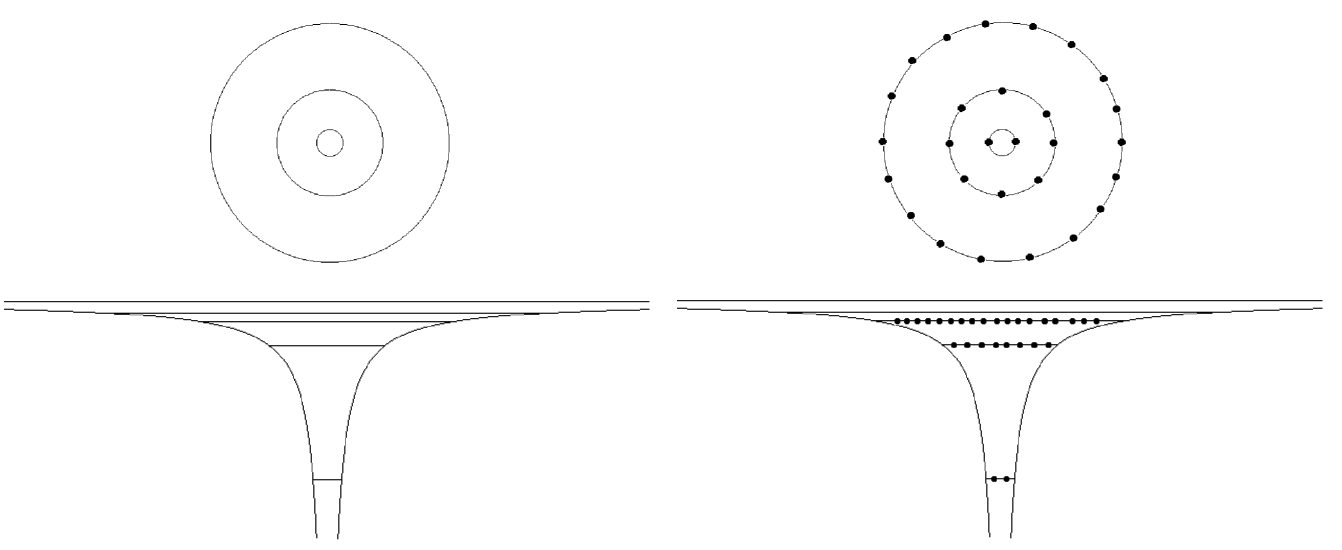
Aufbau__building-up.png
Closed shells do not follow the n2
rule 1s 2s 2p
3s 3p
3d 4s
4p 4d
4f 5s
Configuration_orbitals.png
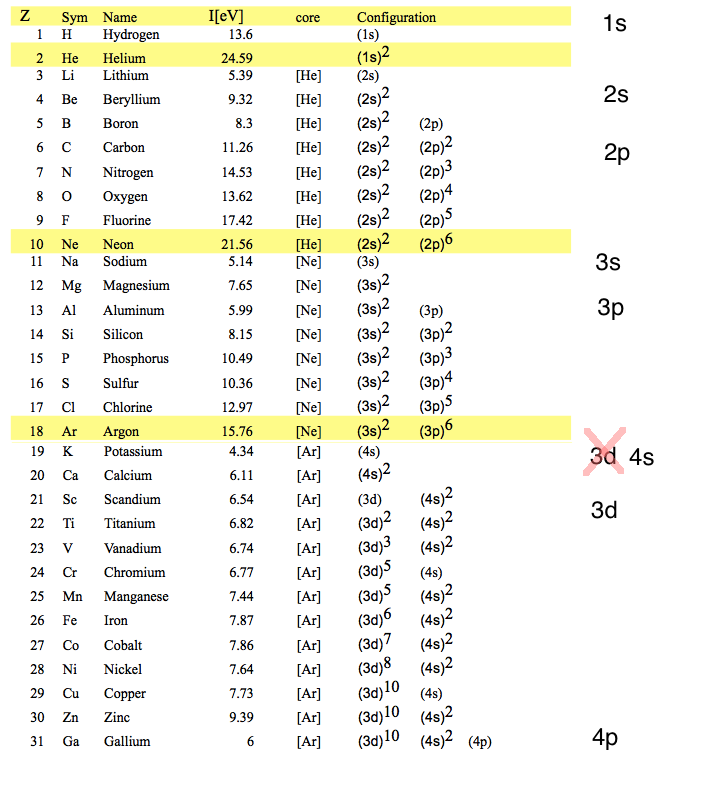
Configuration_orbitals.png
Interaction with a charged
cloud
xcf_0015_electro-potential-point-cloud.png
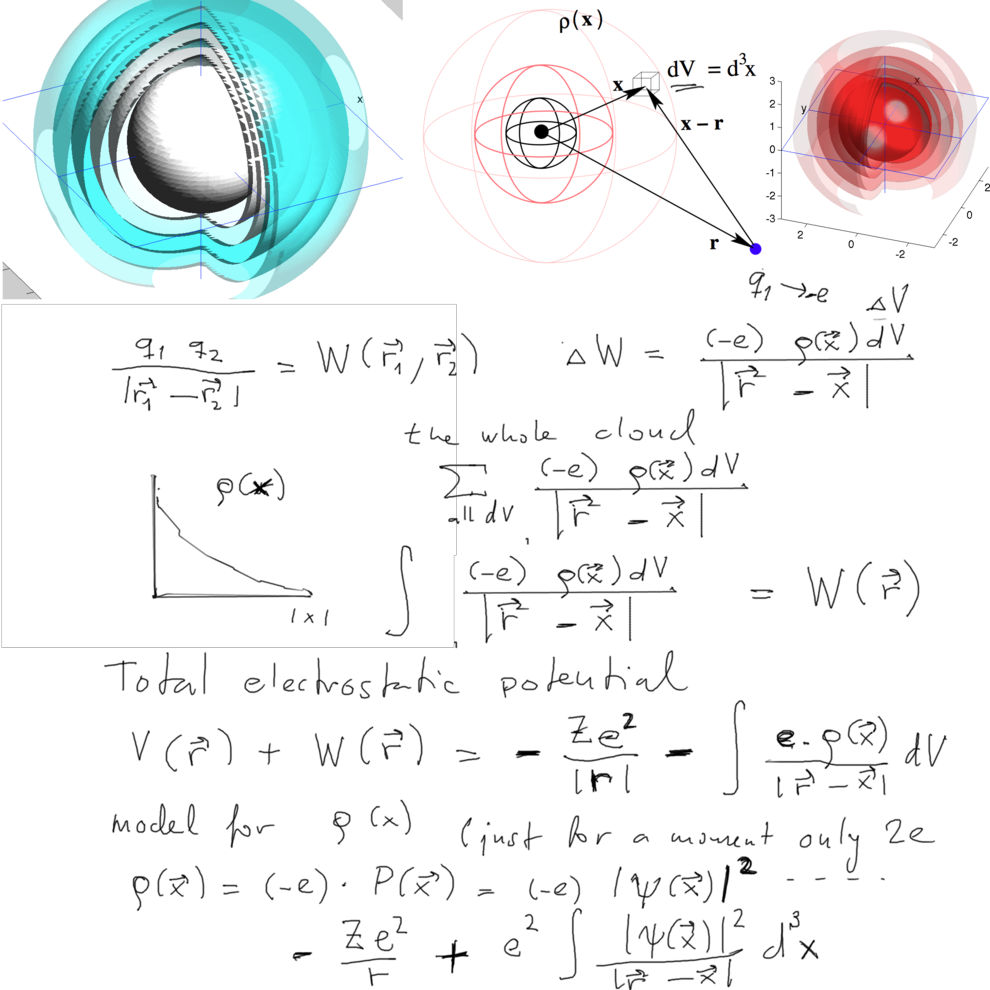
xcf_0015_electro-potential-point-cloud.png
xcf_0020_orbitals_in_cloud.png

xcf_0020_orbitals_in_cloud.png
xcf_0025_ORBITALS.png
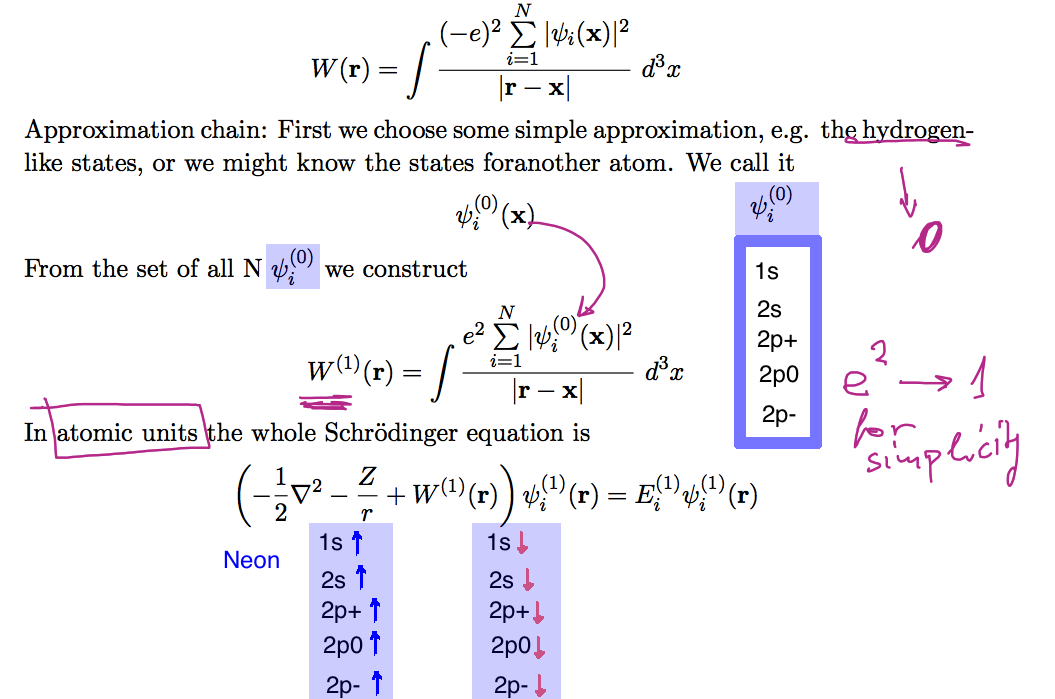
xcf_0025_ORBITALS.png
xcf_0029_ITERATIONS.png
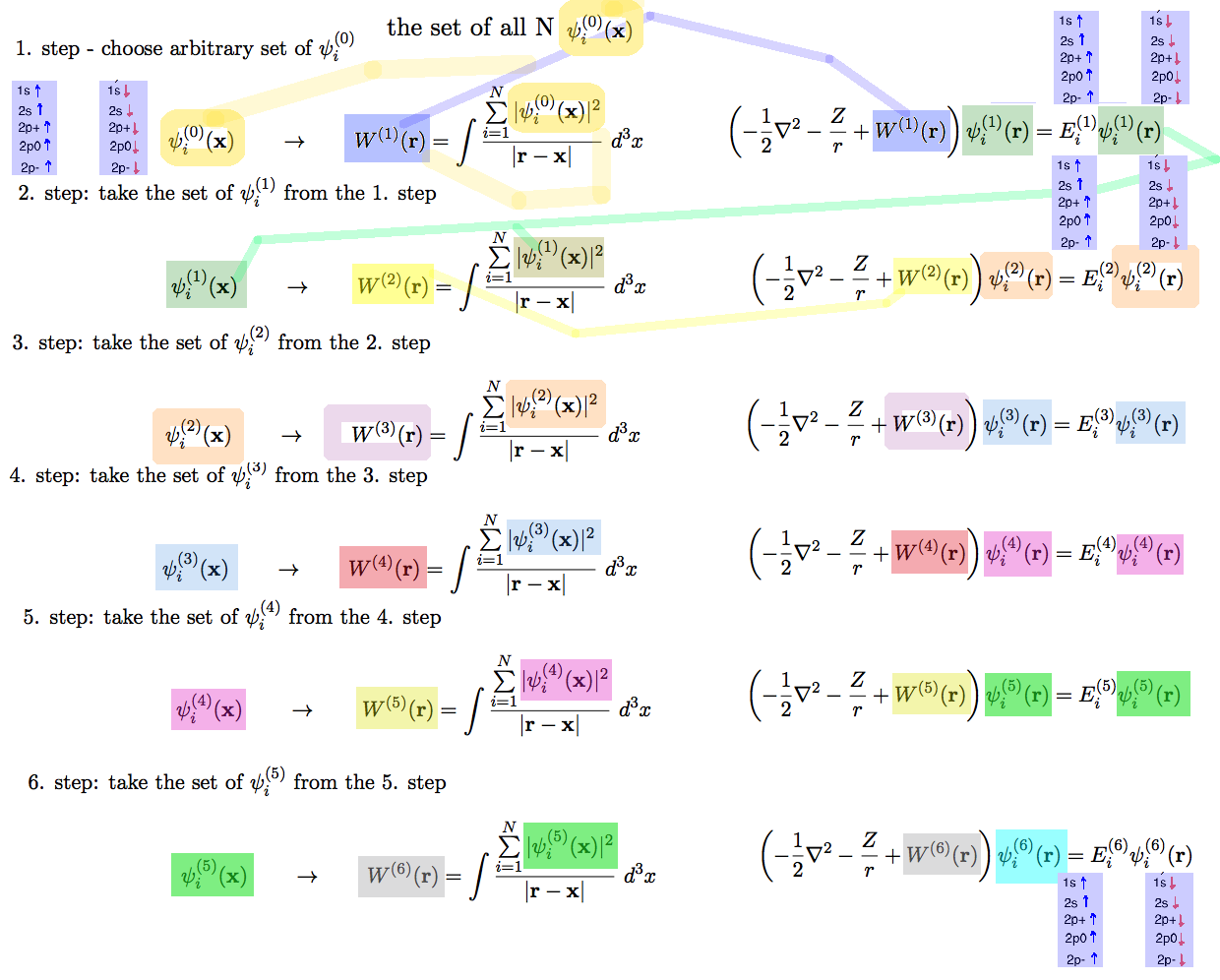
xcf_0029_ITERATIONS.png
xcf_0030_self-Consistent_field.png

xcf_0030_self-Consistent_field.png
ONCE MORE:
Closed shells do not follow the n2
rule 1s 2s 2p
3s 3p
3d 4s
4p 4d
4f 5s
Configuration_orbitals.png

Configuration_orbitals.png
Explanation of the 3d - 4s anomaly
(deviation from the n2
rule )
- Interplay of
screening and centrifugal potential
Here we compare a model for the screen potential (below) with the
Coulomb n2
rule degenerate (hydrogen-like) - UPPER PART
coulomb_vs_Hartree.png
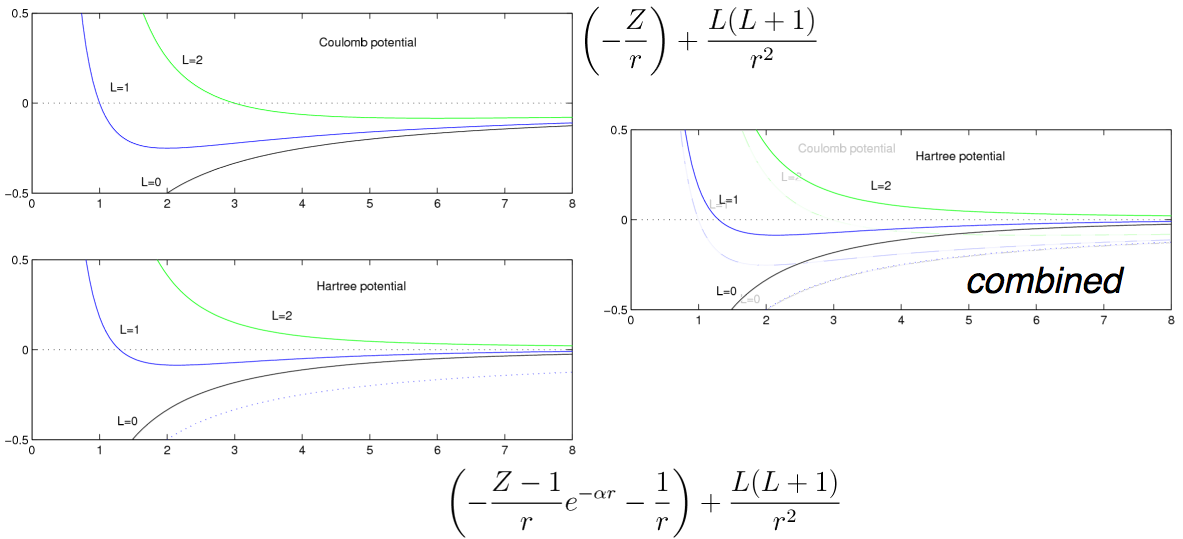
coulomb_vs_Hartree.png
Results from the computer program
../2013_10_10/#computing
but mainly ../2010.10.14/
and the links there
xcf_0040_Program_to_do_Self_consistent_field.png
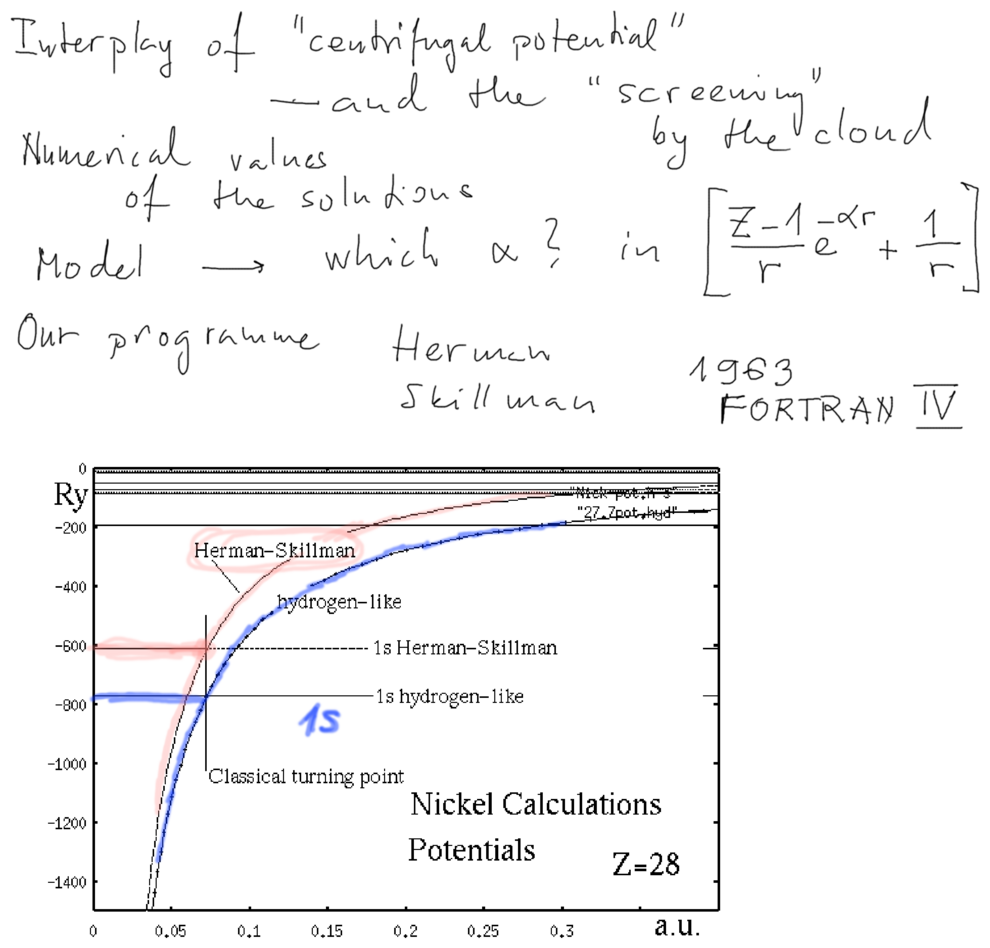
xcf_0040_Program_to_do_Self_consistent_field.png
Results from the computer program
../2013_10_10/#computing
but mainly ../2010.10.14/
and the links there










