Many Electron Atoms - part 2
Evaluating expectation value of total H
- similar purpose as in the He case:
it is t be used finally in a variational approach
Using product function - where each electron placed in its orbital
(Pauli principle must be obeyed)
Generally the situation is more complicated for n > 2
because one can not make antisymmetric function of three spins
If we think that we allways work with j-j coupling, the situation is
much simpler
( orbitals characterized by n,l, j and m of j - see the discussion
further on )
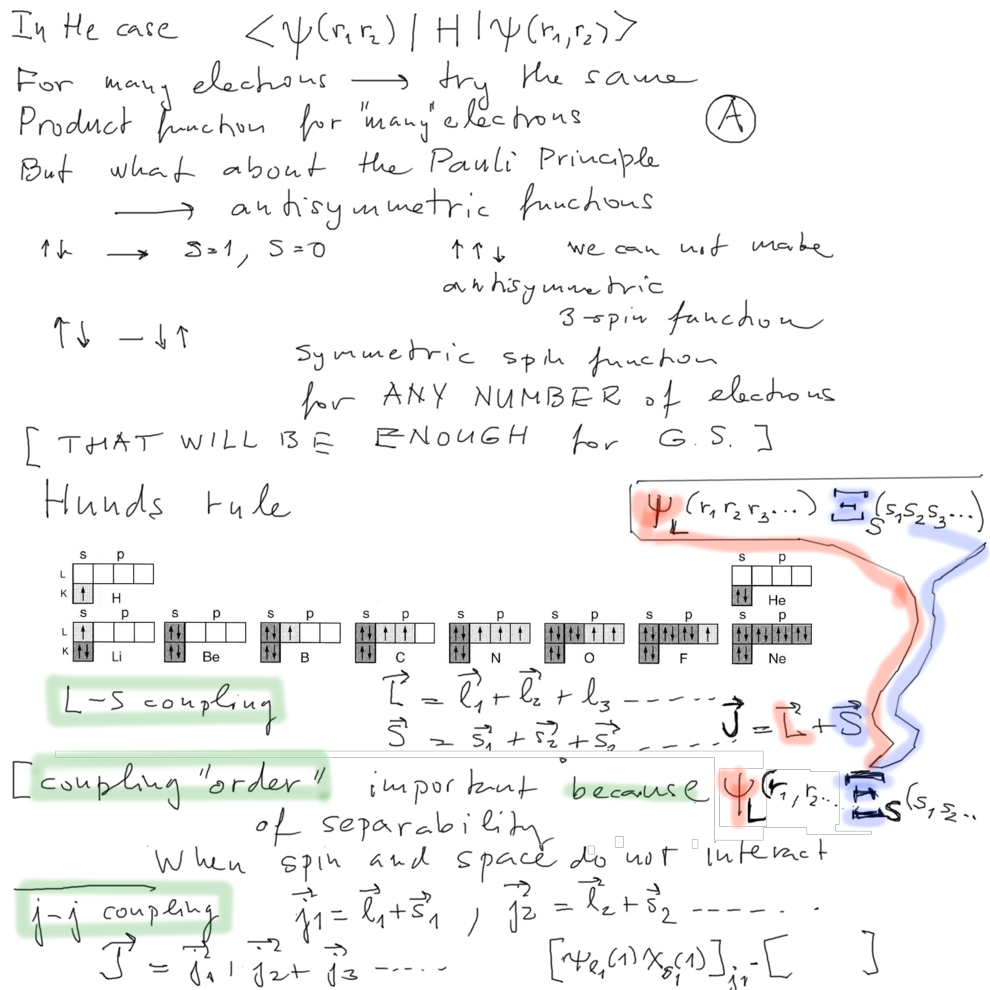
Hund's rule: the levels
are filled so that maximum spin S is achieved IN THE GROUND STATE
That means - the spin function is SYMMETRIC
which means the space function is antisymmetric - THE
REPULSION is weakest possible
-
the story of orthohelium once more -
but in Hund's times
this was not understood - therefore a special Hund's rule
xcf_0011.png
xcf_0011.png
Hund's rule is historically important and popular in introductory
texts:
http://hyperphysics.phy-astr.gsu.edu/hbase/atomic/hund.html
More on L-S and j-j coupling - See the next board.
What does it mean that 2 angular momenta interact? Probably that
the total energy of the system
depends
on
mutual orientation of the
two VECTORS - i.e. of
their scalar product
H = H0
+ J1 . J2
where
J1 and J2
are the 2 angular momenta H0
is
all the other energy
(
remember the effective spin-spin interaction, He ? )
xcf_0020.png

xcf_0020.png
How to make antisymmetric (product) functions for N particles
( already N=2 and larger) ??
The answer: REMEMBER THE DETERMINANTS - antrisymmetric
property
permutations ( http://en.wikipedia.org/wiki/Determinant
)
xcf_0025.png

xcf_0025.png
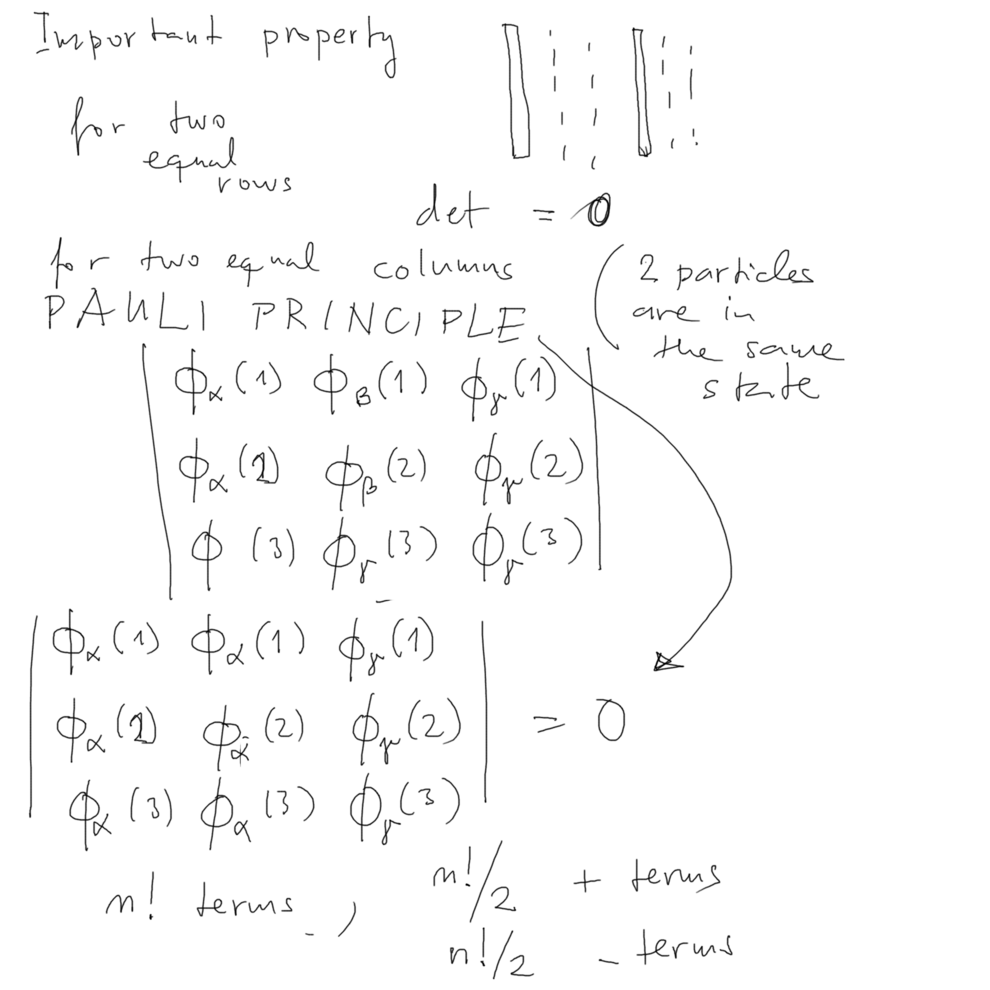
From the athematical definiton of the determinant - one property
immediately leads back to PAULI:
NO TWO COLUMS CAN BE EQUAL - and no two rows can be equal
xcf_0029.png
xcf_0029.png
Look once more:
From the athematical definiton of the determinant - one property
immediately leads back to PAULI:
NO TWO COLUMS CAN BE EQUAL - and no two rows can be equal
in our choice of notation no 2 columns IS RELEVANT
Normalization - there are N! terms - this gives the
normalization factor each term gives 1
product with other terms gives 0 - thus ( one over squ
xcf_0032_Single_Particle_Operators.png

xcf_0032_Single_Particle_Operators.png
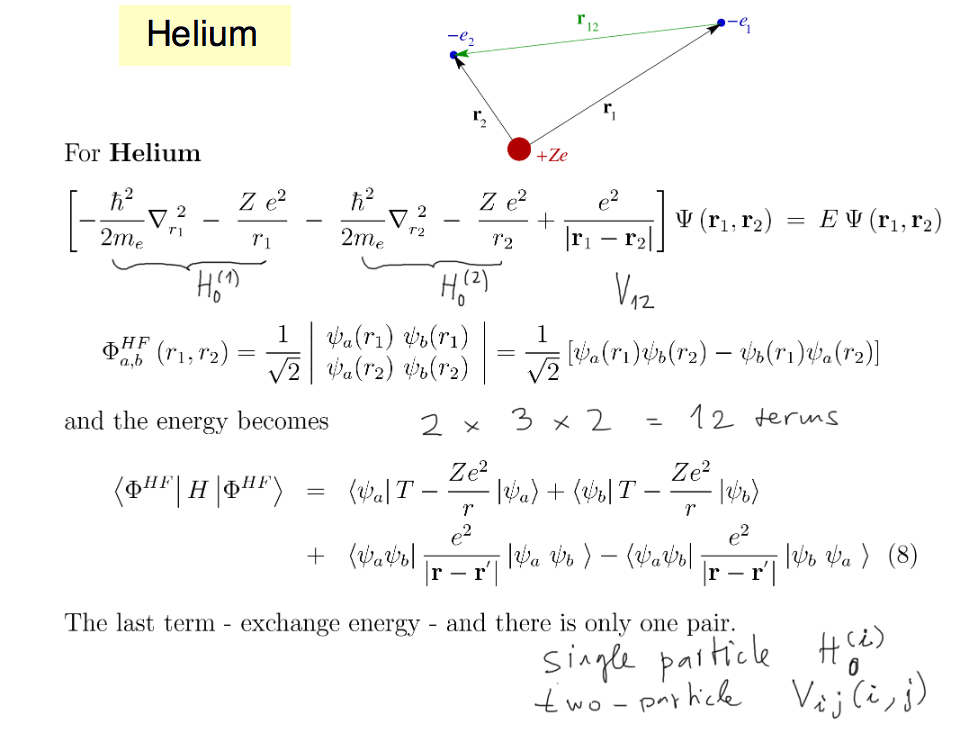
In the case of Helium, we already
discussed this - how the terms get reduced in number (they are
equal, and the normalization)
The discussion is reprinted below
xcf_0034_Helium.png
xcf_0034_Helium.png
xcf_0035_Lithium.png

xcf_0035_Lithium.png
nonzero-terms-Pair_interaction_operator.png
nonzero-terms-Pair_interaction_operator.png
THIS
IS
REPRINTED
FROM ../2014_09_18/index.html
-
discussion of exchange
interaction in the Helium case
jump
BACK
to
the Helium - Lithium discussion
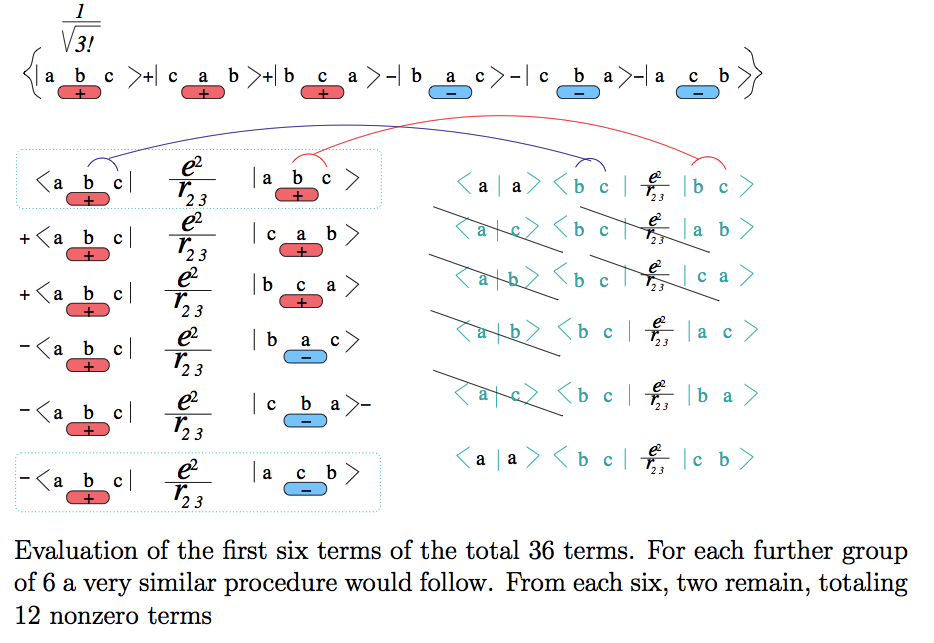
How
does
it go - the
2
times 3 times 2 = 12 terms become just 4
terms !
Look here - we did it at the
whiteboard:
xcf_a050.png - from 28.09.2014
------------ 
xcf_a050.png - from 28.09.2014
The main aim of the present part is to prepare the variational method
- which in turn will lead to a
formal derivation of self-consistent field - SCF - method








