Units suitable for the everyday life are not suitable for description of astronomical objects, and they are also
little suitable for the world of atoms and molecules. So the units are important, in spite of what some theoreticians
from time to time claim, for example introducing "natural units" which then "remove" all the "unimportant" constants.
First we look at the units, then we start our approach to the physics of Helium and two-electron atoms.
This unit of length can be obtained by various arguments - some are shown in the first slide note.
The Bohr radius

0094_Atomic_units.png
When dealing with energies, half way to suitable units is to use the ELECTRONVOLT - 1 eV - as a useful unit.
This is because the electronvolt is used in many connections ( warning: the chemists use a unite close to 1 eV,
but different. It is called kiloJoule per mol
1 kJ/mol -> 0.010364 eV (per particle)
1 eV -> 96.48 kJ/mol ( 23.06035 kcal/mol )
Note that the Coulomb energy expectation (27.2 eV), and not the H-atom binding (13.6 eV) is chosen for energy unit
(some people did just that, using 13.6 eV; this mixes things up a bit, see below, the Wikipedia case)
We calculate below the convenient atomic unit of time : it is 25 attoseconds - or 0.025 femtoseconds

0098_atomic_units_energy_time.png
Below - the confusion which can result from a "seemingly convenient" choice.
The difference is what one selects - should we have a 1/2 in the definition - or not.
The below quoted writers take this literally as two different choices - an absurd story results,
- who claim that these two choices "differ in the choice of the unit of mass and charge
https://en.wikipedia.org/wiki/Atomic_units
Below is shown that just the 1/2 is sort of misplaced.
The natural units "equation" is another absurdity often found in print .....

0099_Hartree_Rydberg_wikipedia.png
Helium and two-electron atoms.
Six coordinates, 2 kinetic energies and THREE potential energies;
One part of the potential energy is the electron-electron repulsion.
Last time we worked with VARIABLE SEPARATION into two ( independent - if lucky ) equations.
In He - not possible, due to the repulsion, which "mixes" the variables
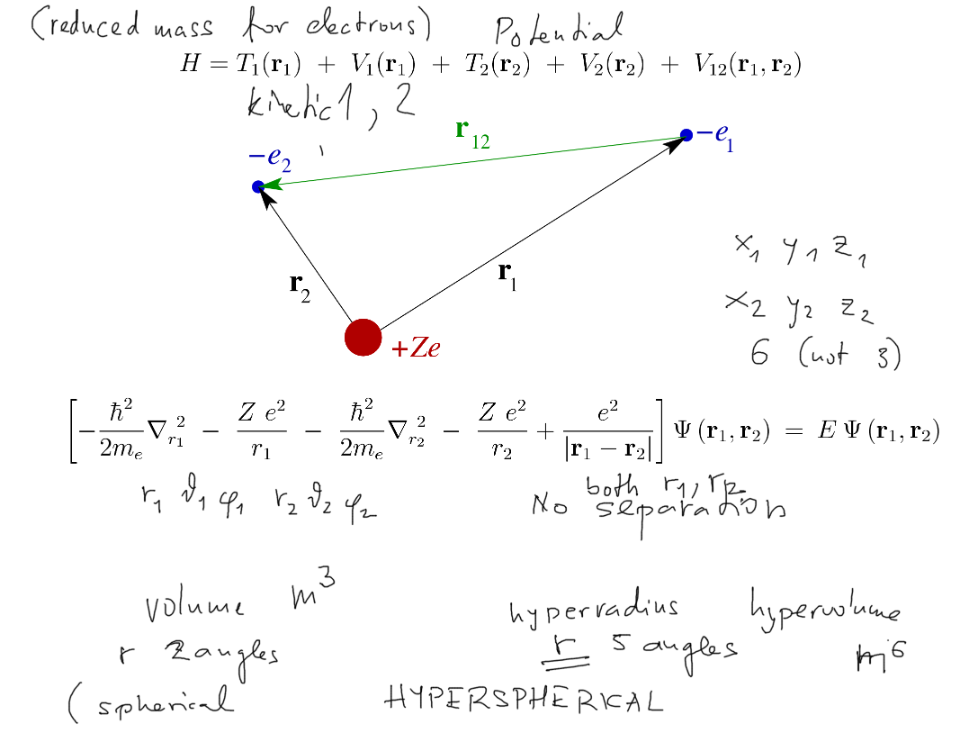
0100_He_coordinates_Hamilton.png
Approximations necessary
we start by - for a while - neglecting the "complicating" electron repulsion.
Then we can do the separation - assuming that the solutions are products of function of each variable
Product --> independent particles -- as we shall discuss next time
With the separation -- approximation that each of the two electrons obeys
its own hydrogen-like Schrödinger equation
(two "parallel hydrogen atoms")
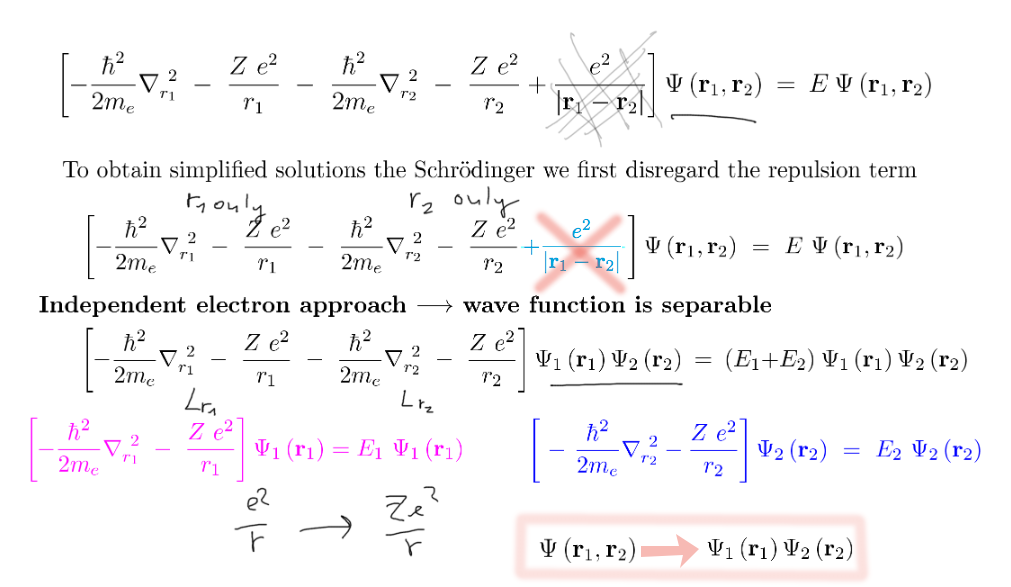
0110_Independent_electrons_Separation.png
The energies are shown below (together with better approximations)
We see that this idea fails badly.
Total binding in He ground state is 79 eV, it takes 24.6 eV to make free the first electron (singly ionize), and the
second is hydrogen-like - to remove that is 4 times 13.6 eV --> 54.4 eV, thus the total 79 eV (experimental results)
Our "approximation" give twice 54.4, it means 108.8 eV.
We shall improve this next time, when we shall try to include the electron-electron repulsion in a somewhat better
version of outr approximation
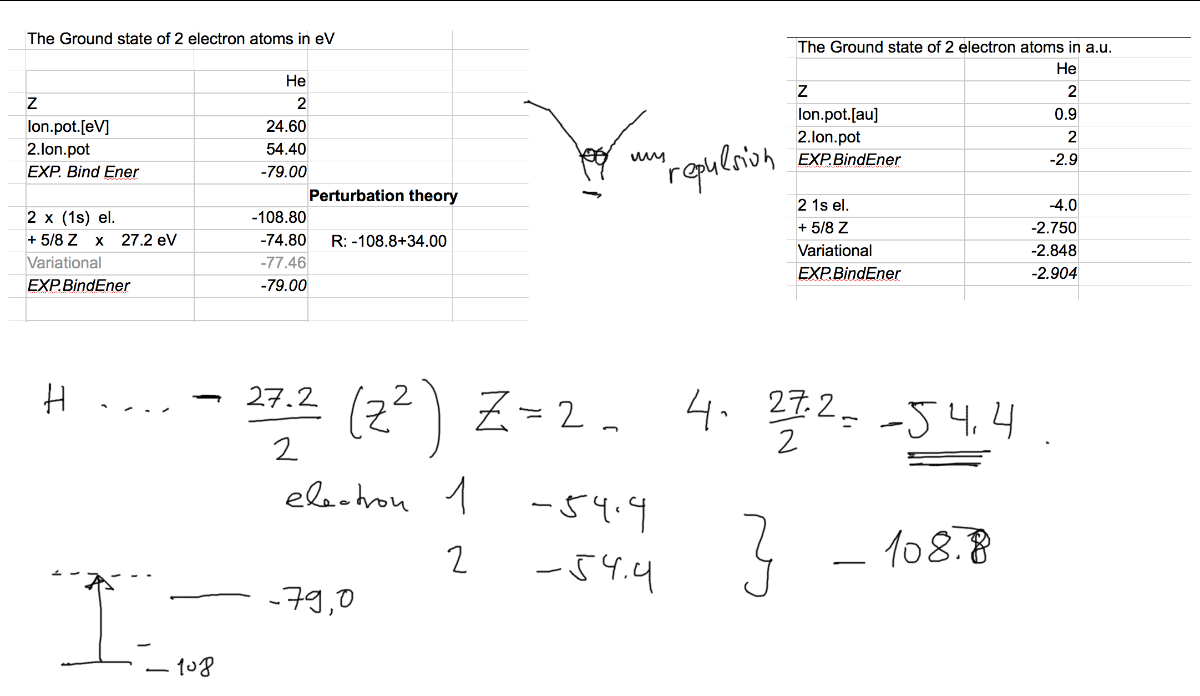
0200_n_Energies_Approximations.png
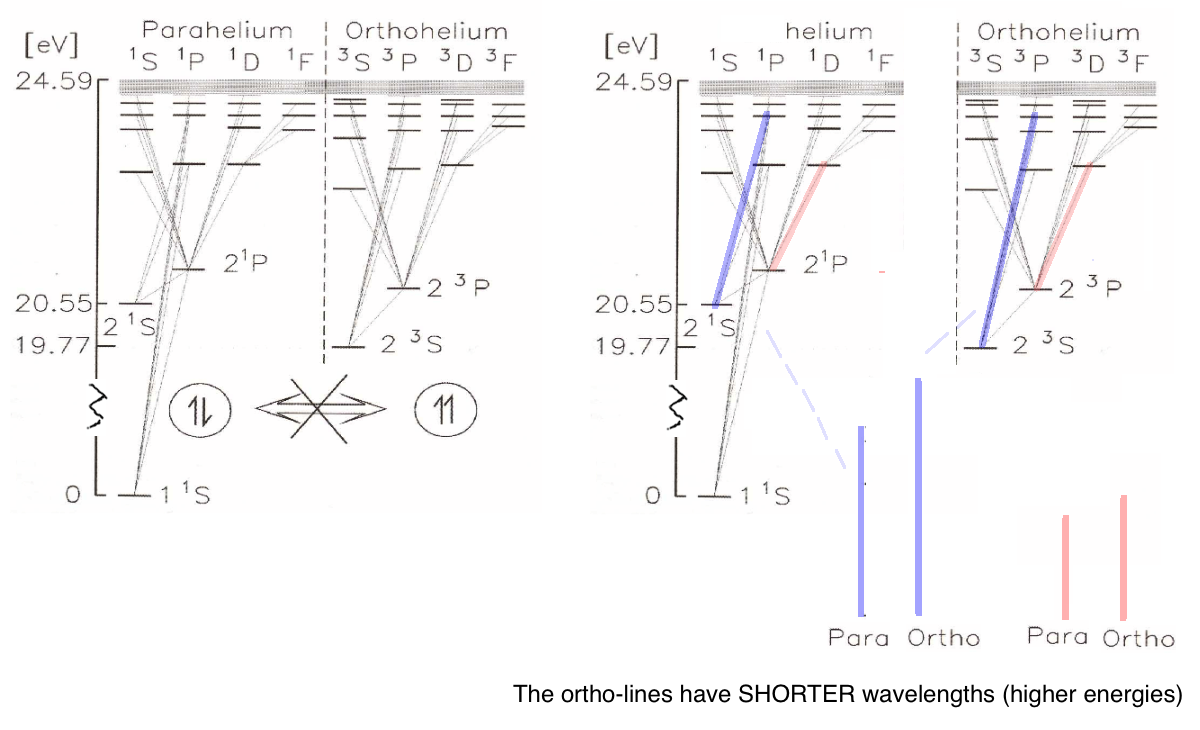
The old experimenters actually believed there were two "versions" of helium, which they called
orthohelium and parahelium (quite confusing names); but one should add that they were looking at
the transitions close to visible ... more on this when we treat this in detail
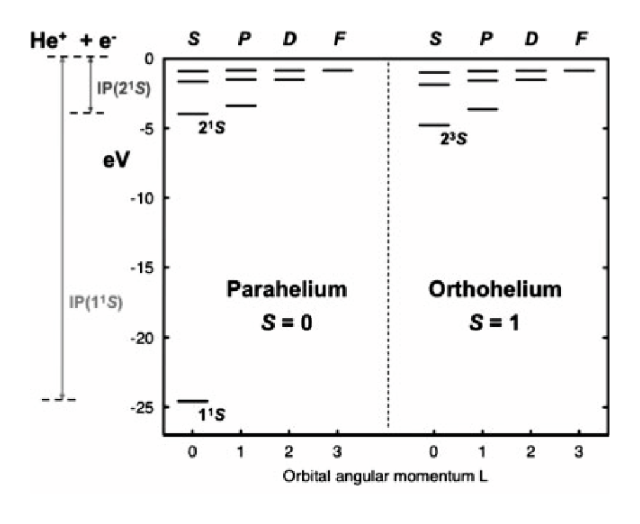
0300_ortho_para_helium.png
can not be really accurate - here we have the levels in some more detail.
Note again that the spectroscopist only "sees" the transition lines (wavelengths). The level schemes must be constructed.
0320_ortho_para_helium.png
Next time we continue with the GROUND STATE - starting with the electron repulsion calculations.