extension - and reformulation - of the Perturbation theory (for repulsion) discussed before.
Then we started with treatment of EXCITED STATES (i.e. not the GROUND STATE).
Then we started with the role of ELECTRON SPIN
kinetic, potential and electron repulsion terms, showing their Z-dependence.
What is variation, Calculus of variations?
wikipedia says: en.wikipedia.org/wiki/Calculus_of_variations
Calculus of variations is a field of mathematical analysis that deals with maximizing or minimizing functionals.
Functionals are mappings from a set of functions to the real numbers
( as Functions are mappings a set of numbers to numbers)
We shall se why A MINIMUM of a FUNCTIONAL is important in Quantum Physics
( but also in many other physics applications )
How to get to a 'set of functions' in our case - see bottom of the plate -
each electron 'sees' unknown Screened z instead of Z of the nucleus

0000_perturbation_Functionals_variation_from_mapping.png
Theorem The exact solution for the GROUND STATE gives the lowest energy expectation
Proof next plate - for any system

0010_Ground_state_extremum_expectation_value_Hamiltonian.png
1. Find the set of possible solutions
2. Find the minimum of the FUNCTIONAL ( i.e. the expectation value of the hamiltonian operator)
This function is then "closest to" the real solution
Below we thus see that the best screened z is given by z = Z - 5/16 ( or about z = Z - 0.3 - for all Z )
i.e. the "best independent electron approximation"

0030_result_variational_z_from_Z_for_2-electrons.png
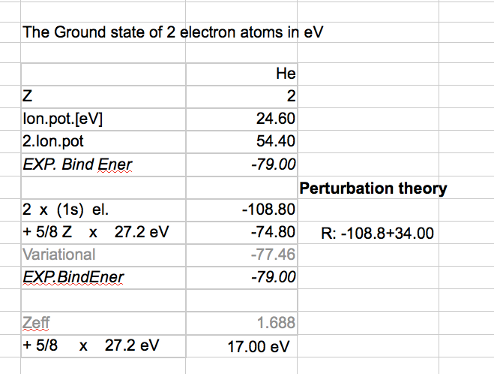
0035-spreadsheet-energies.png

0037_TABLE_all_and_variational_six_Z_for_2-electrons.png
Configuration mixing will be visited several times later

0040_NOT-independent_correlation_Configuration_mixing.png
Functions where electrons are NOT INDEPENDENT - not a product ( Hylleraas 1930 )
en.wikipedia.org/wiki/Egil_Hylleraas
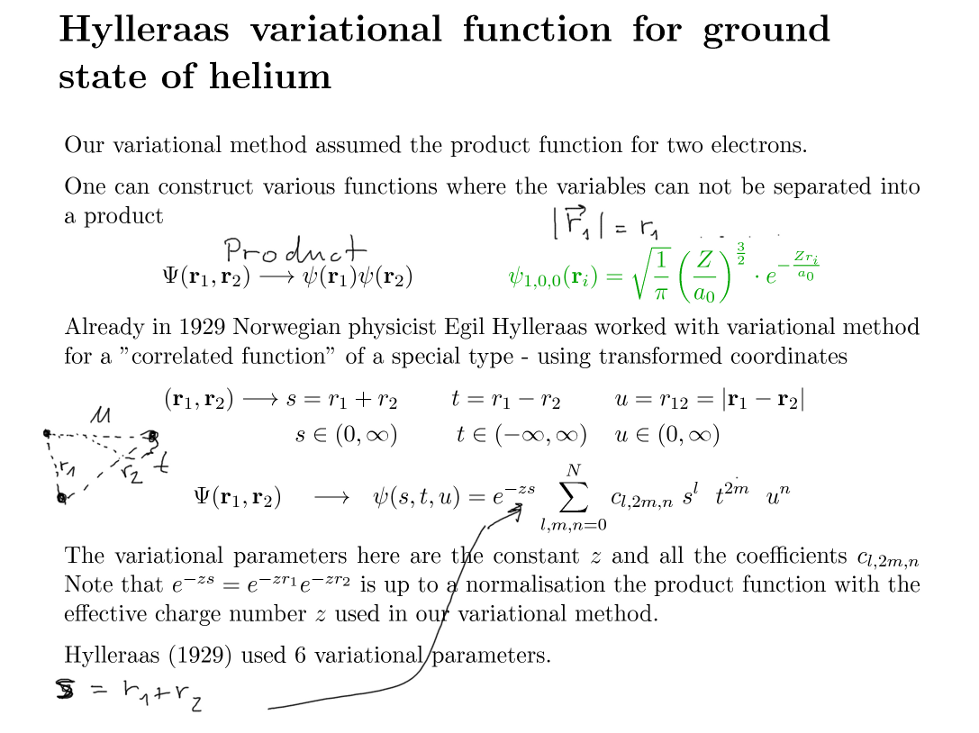
0050_Hylleraas_Variational.png
Excited states, the role of the spin
Excited states - one electron is in n,l state instead of 1s - we call this singly excited.
Also here the repulsion, configuration mixing etc will play a certain role
- but we look mainly at the role of exclusion principle (Pauli) - spin has "two possible eigenstates"
What is spin? Not really "angular momentum"
Postulated by Pauli and contemporaries; Derived from Dirac equation - it is mainly an extra (internal)
magnetic moment
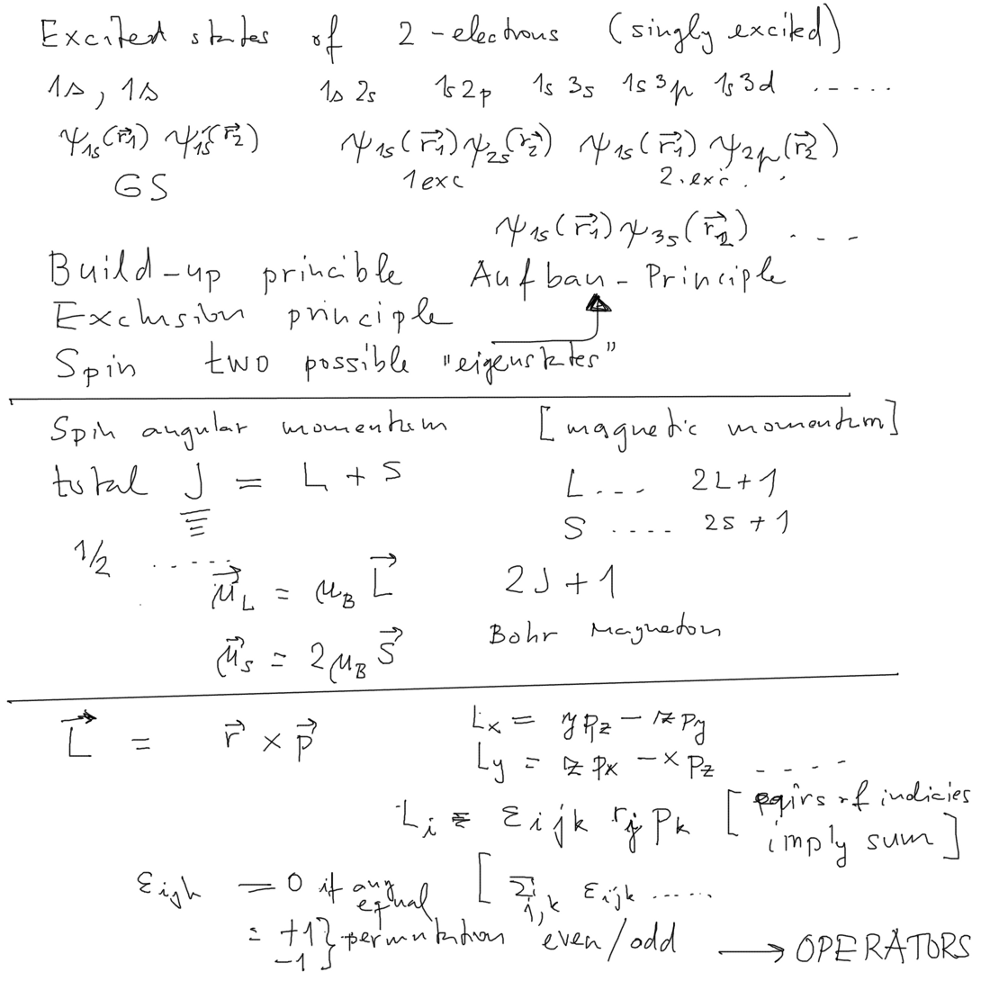
0060_Helium_excited_states___Aufbau__Angular_momentum_Spin.png
Commutation rules - spin operator was found to obey the same rules - Pauli matrices
Comment: we have forgotten to look into Pauli Matrices here - it will be visited later
"Exchange symmetry" - permutations and all that mentioned - next time
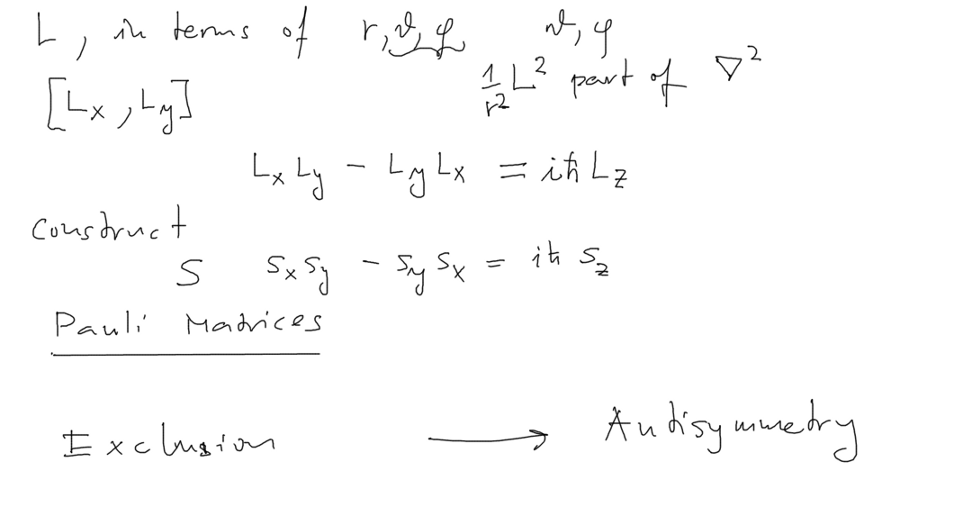
0070_Angular_momentum_Spin_exclusion_principle.png
"Exchange symmetry" - permutations and all that mentioned - next time