And addition of angular momentum mechanisms
Two types of helium spectra - Orthohelium, Parahelium. The origin of the energy differences
Doubly excited states of Helium (this will not be of interest for other 2-electron systems - why? )
Configuration Mixing - from the expansion theorems.
en.wikipedia.org/wiki/Magnetic_moment Long article on magnetic moment, definition etc
en.wikipedia.org/wiki/Magnetic_moment#Magnetic_moment_and_angular_momentum
en.wikipedia.org/wiki/Magnetic_moment#Magnetic_moment_of_an_electron

xcf_0000_Magnetic_Moment.png
( the spin 'wavefunction' is not really a function as e.g. 1s state radial; for us it can be a 2x1 (column) matrix )
Next plate: We find many illustrations of addition of angular momenta - from 'sort of everyday life'
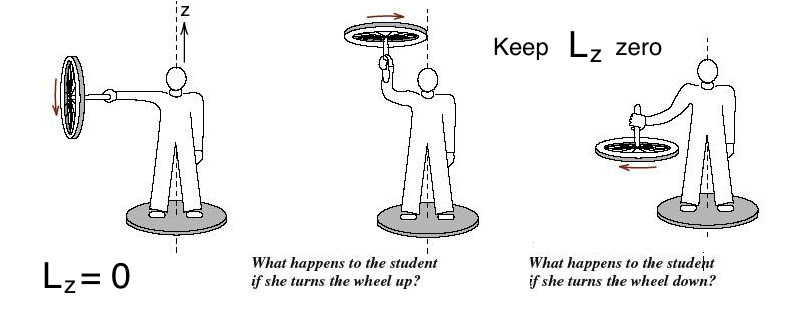
xcf_0005_addition_angular_momentum.png
Addition of angular momenta - see the trianle rule below
the Aufbau Principle --> Exclusion Principle (Build-up Principle)
Exclusion Principle justified by Fermi Statistics
identical particles --> Invariance for exchange of particles
the exchange of positions must not change the PROBABILITY DENSITY ; phase --> plus / minus 1
Antisymmetry - the minus 1 possibility --> leads to exclusion principle
exclusion principle TRUE IF electrons are Fermions

xcf_0010_exclusion_principle.png
Below:
But we must include both spin and space!
And spin and space must be INDEPENDENT
INDEPENDENT ---> PRODUCT wavefunctions

xcf_0020_Fermi_Bose_identical_particles_exchange_symmetry.png
Space ANTISYMMETRIC spin SYMMETRIC
L-S coupling vs j-j coupling
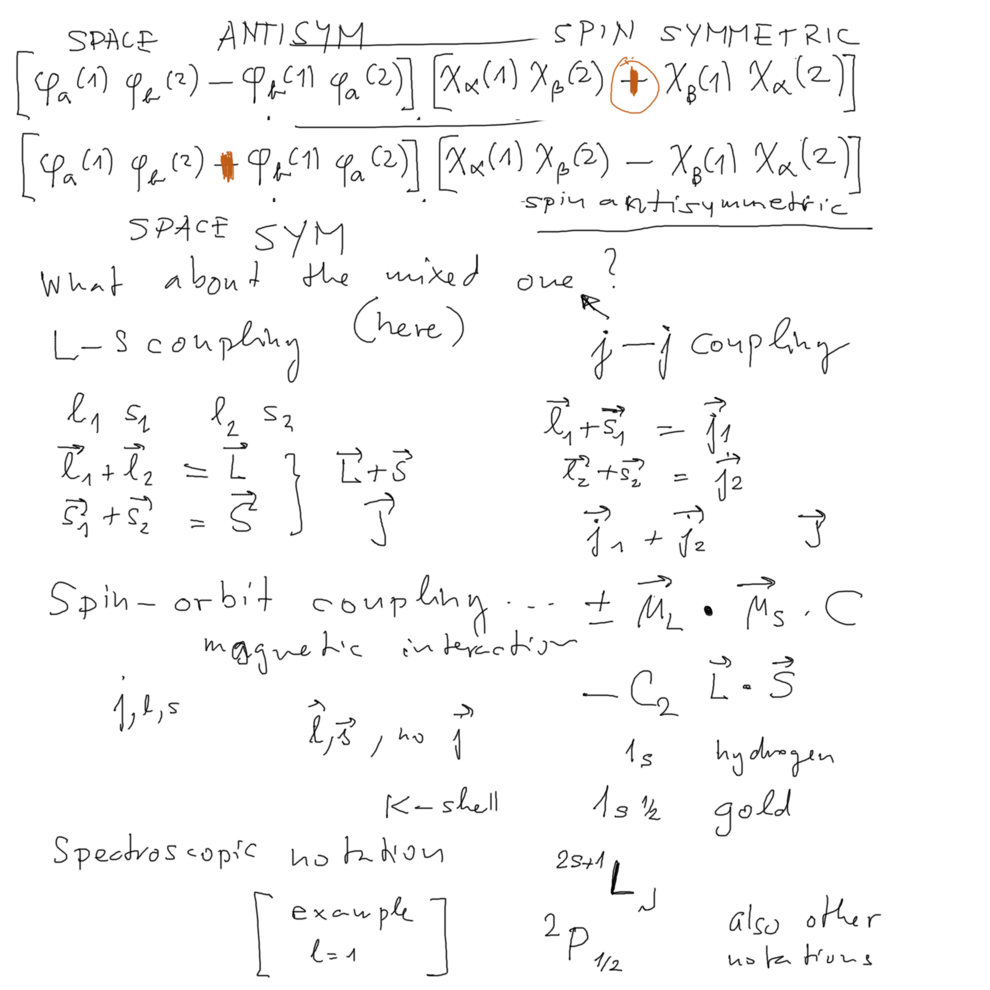
xcf_0030_Exchange_Symmetry_L-S-j-j-couplimgs.png
Below: towards the explanation of the ORTHOHELIUM - PARAHELIUM energy difference

xcf_0035_Triplet_SInglet_SYM_ANTISYM.png
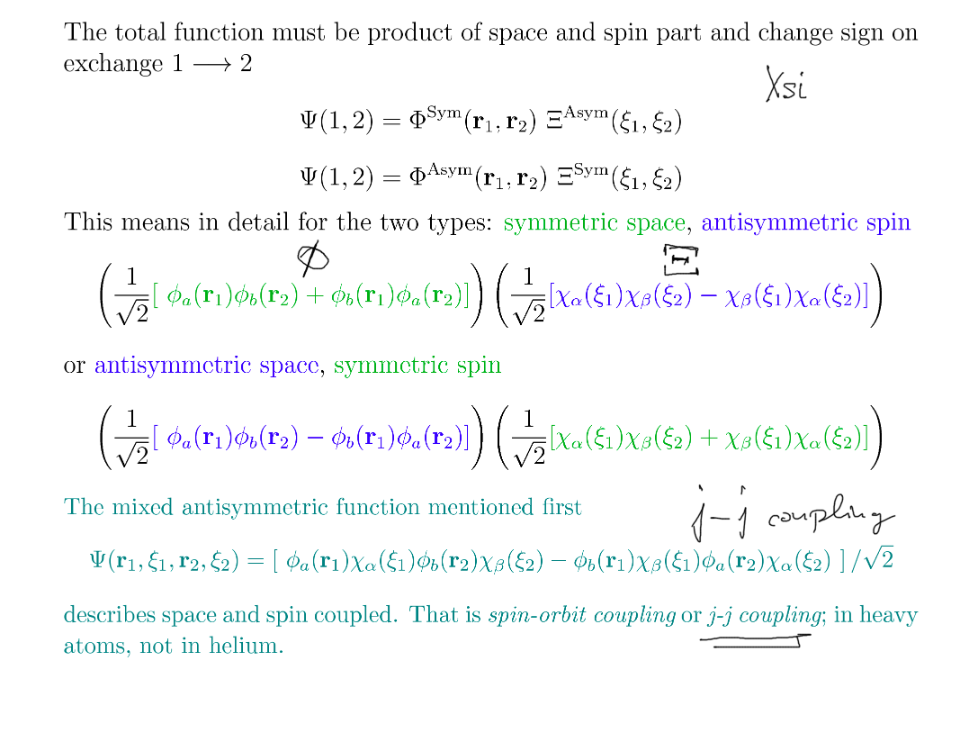
xcf_0039.png

xcf_0040_Triplet_SInglet_SYM_S-1_ANTISYM_S_0.png

xcf_0041.png
triplet S=1 (SYM) singlet S=0 (ANTISYM )
space ANTISYM space SYM
repulsion reduced, small repulsion LARGE
ORTHOHELIUM below PARAHELIUM

xcf_0043_Symmetric_Antisymmetric_Spectra.png

xcf_0100_Doubly_excited_States.png
Doubly excited states - detailed energy levels
This energy level diagram shows the binding energies of the ground state,
ortho-helium, parahelium singly excited states, the ionization threshold state and
the spectrum singly ionized helium - and the threshold for doubly ionized He - the alpha-particle
The electron repulsion "pushes" the doubly excited states of He to regions above the
single ionization threshold ( see diagrams on autoionization below )
so that all doubly excited states are autoionizing states
( this is not allways the case for other Z than 2, but very energy relations shown below are valid )
A de-excitation via an autoionization is often calle Auger process or non-radiative transition as opposed to radiative deexcitation
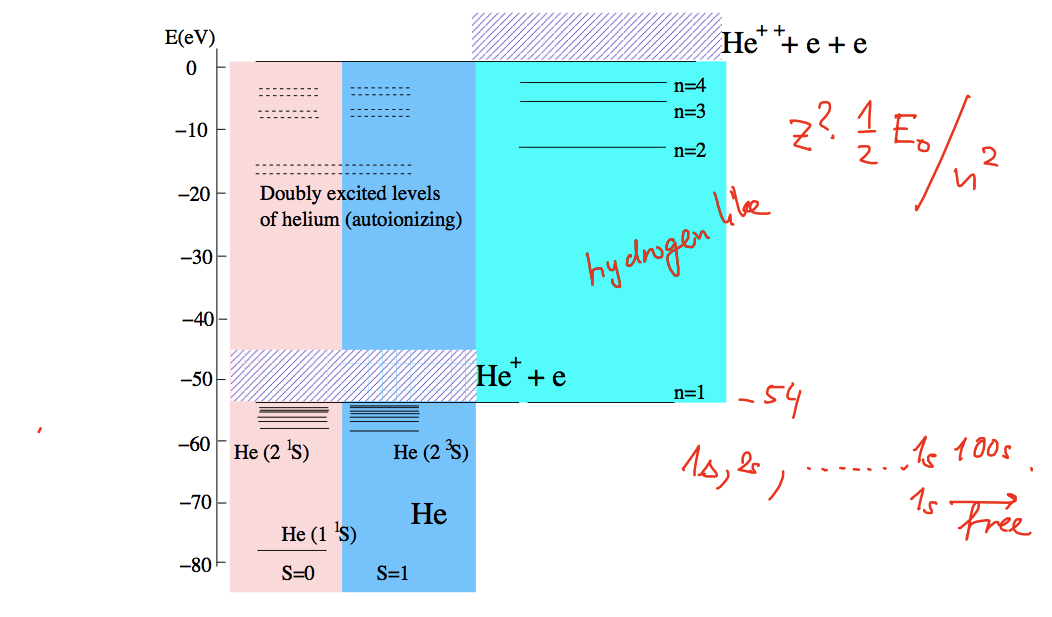
xcf_0110_Doubly_excited_States.png
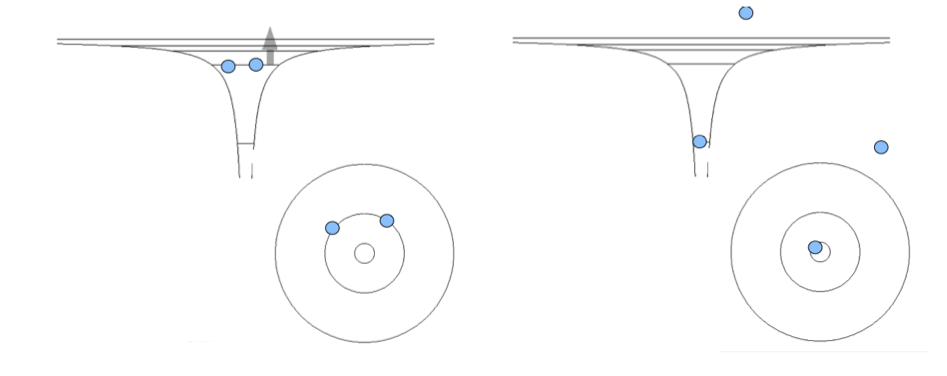
xcf_0115_Doubly_excited_and_de-exciting.png
The energy is conserved;
We see that the sum of energies in the second and third picture
are EQUAL. Therefore the system can go from one to the other state - no energy involved
The first picture - to get there, we must somehow excite 2 electrons at the same time
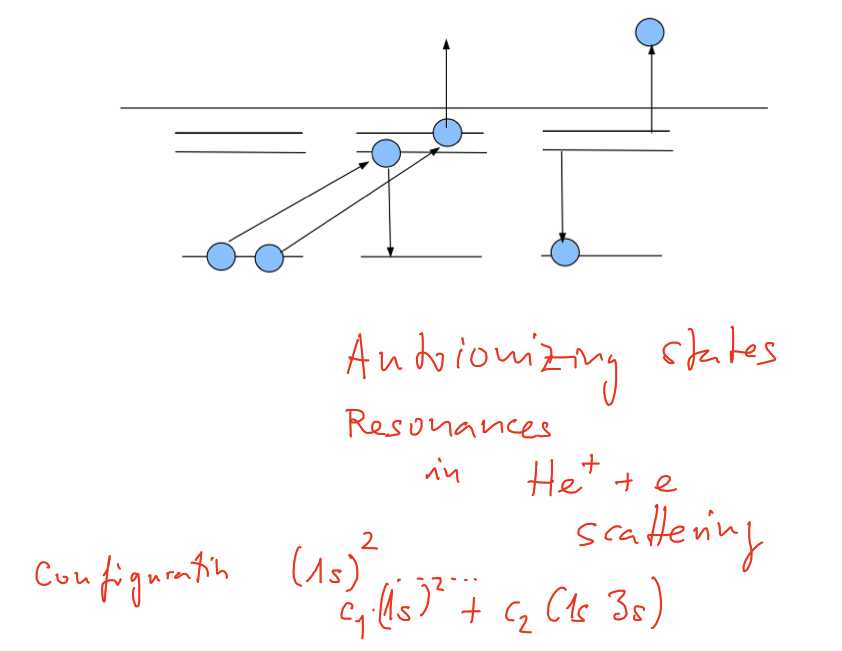
xcf_0130_Autoionizing_states.png

xcf_0200_Configuration_mixing_from_expansions.png
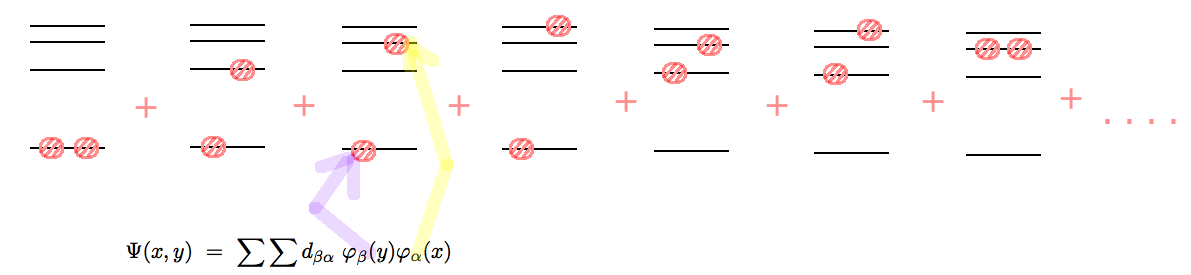
configuration_mixing.png
For atoms, molecules, solids, Nuclei etc ....