Many electron atoms start at a blue region below (link) Some slides got lost - replaced by reconstructions
and last year versions ../2014_09_23/index.html
Extra on spin
Pauli matrices - (up to the hbar/2 ) represent the "spin angular momentum"
The same commutation relations as angular momentum
... see also the vector product of the operator L by itself <-----> commutation relations
Magnetic moment and angular momentum / spin magnetic moment
Addition of two spins Addition of two angular momenta

00010.png
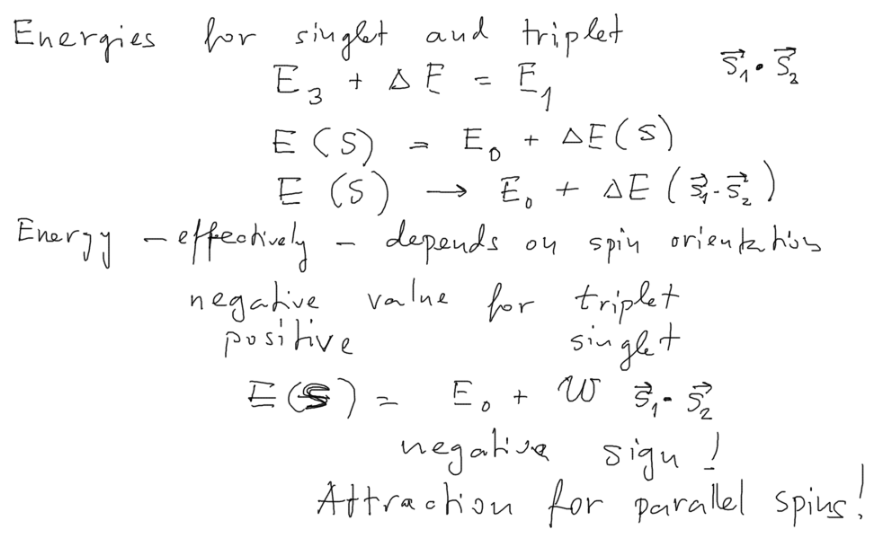
Under : Singlet and triplet energies - Effective spin - spin interaction
00020.png
Singlet and triplet energies - Effective spin - spin interaction
since there is different energy for two values of "total spin" - S=0 and S=1
we can try to write the energy as function of the SCALAR PRODUCT
- i.e the ANGLE between the 2 electron spins
It thus becomes proportianal to the relative orientations of the spins
.... in this case parallel or anti-parallel
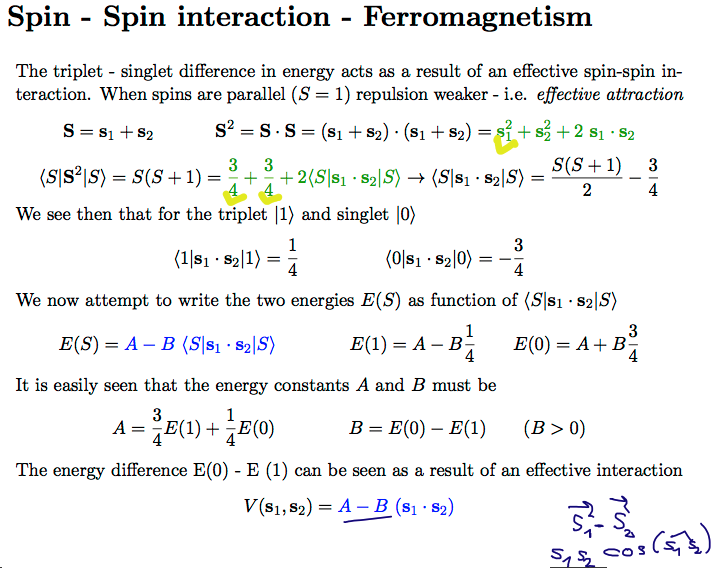
zz_effective_spin_spin_interaction.png
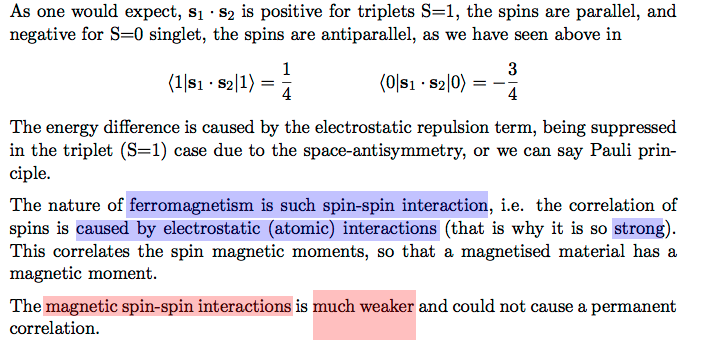
The exchange interaction - leads to effective spin - spin alignment ( minus sign )
zz_z_effective_spin_spin_ferromagnetic.png
Thus understanding exchange interaction / effective spin spin in He ===> Nature of Ferromagnetism
Also: spin - spin alignment - effective spin - spin -- SEE HUND'S RULE in the foollowing part
Many Electron Atoms - Part 1
Hund's Rule - spins want to be parallel ==> effective spin-spin interaction; cf. Helium triplet (orthohelium)
Historically, Hund's rules were very important.
For us they are just an example of the "effecive spin interaction - the Helium triplet states,
i.e. the Pauli principle - and thus antisymmetry of the space part
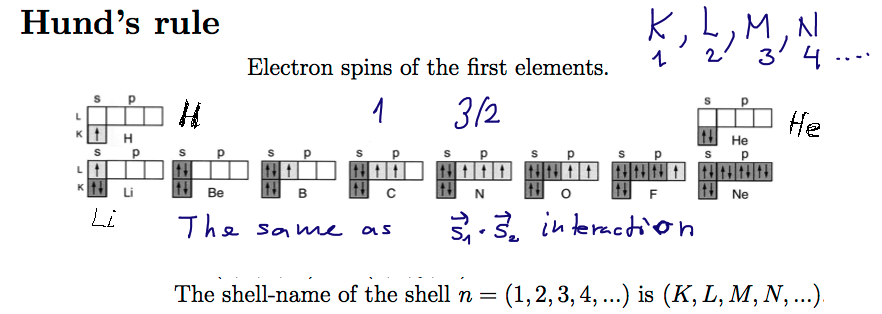
00041_Hunds_Rule.png
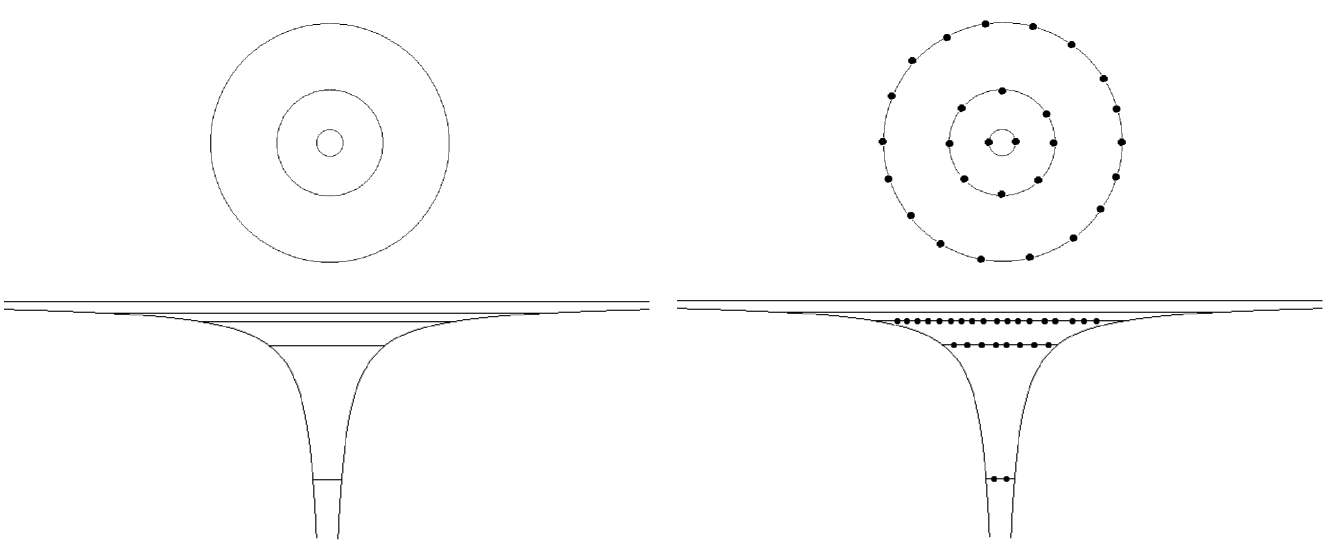
00042_Aufbau__building-up.png
Number of states in n-shell
n l m quantum numbers n l m
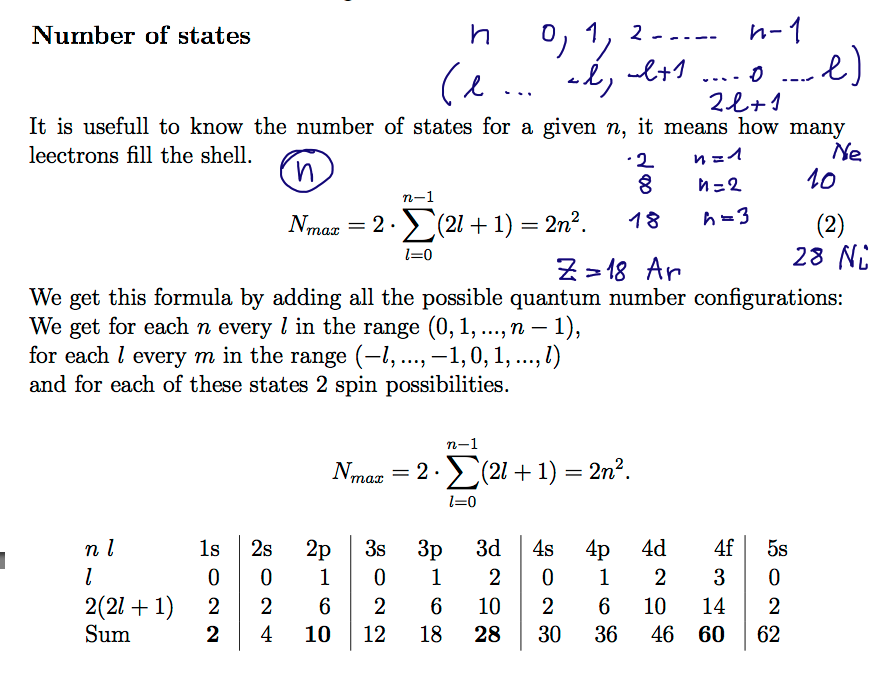
00043_Number_of_states_in_a_shell_N_.png
But the Noble gases are not Z=2, 10, 28, 60; rather Z= 2, 10, 18, 36, 54, ....
Experimental Ionization Potentials ( minus times the Binding energies )
Closed shells - PEAK - i.e. large DIP in Binding energy
WHY - see the sketch in the lower part
Closed shells are NOT closed n - shells --- except for Z=2 and Z=10 Next should be Z=28
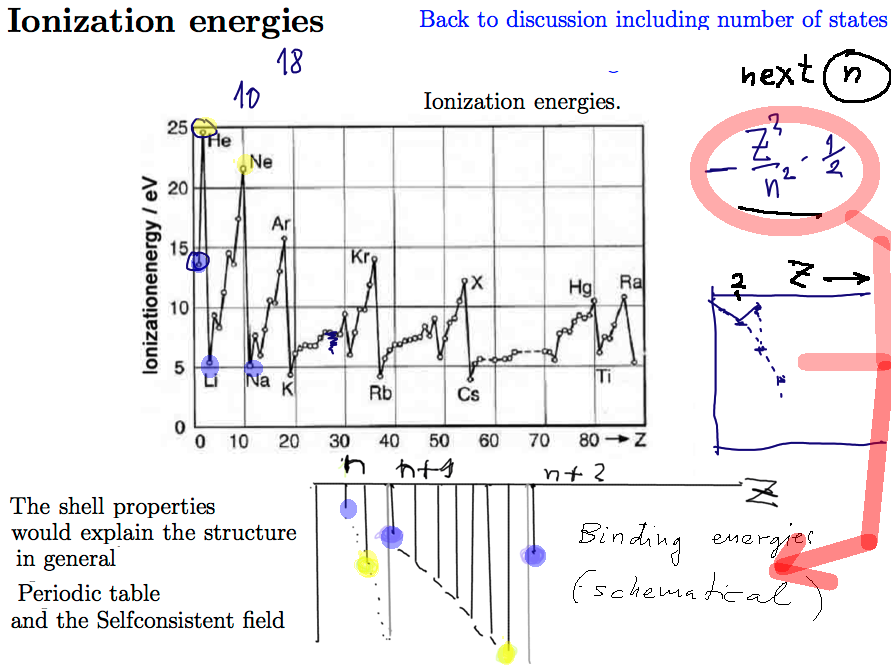
00046_Ionization_Energies_and_Binding_E.png
WHY - - 1/2 Z2 / n2 - --- gets smaller and smaller with Z
until n filled - JUMP up to Z2 / (n+1) 2
Towards the SELF-CONSISTEN FIELD THEORY
Electron cloud - improve the Helium - effective charge by much more flexible "cloud of negative charge"
What is the interaction of an electron with such a "cloud" ?
interaction of an electron with a "cloud" of charge, the charge density, and the charge distribution from the probability
- trying to find which potential energy should be used to describe one independent electron in the atom
(Hartree 1926- 1930s)
Here WE HAVE LOST ORIGINAL - THIS IS A RECONSTRUCTION
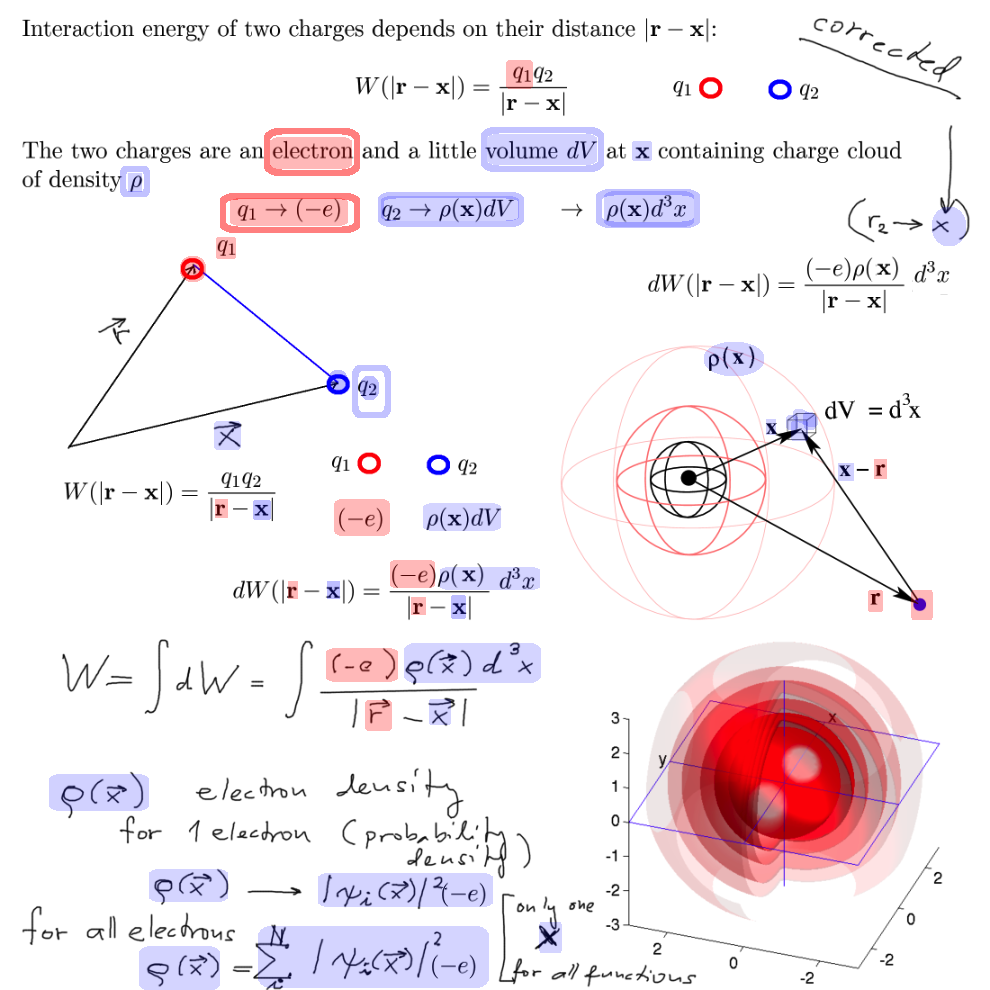
00050_two_charges_dq1_dq2.png
The density (rho) coming from the orbitals, but the orbitals are found
solving Schrödinger equation with the potential resulting from the density (rho)

00060_Interaction_with_the_CLOUD.png
Electron density from probability density ... from wavefunctions ( orbitals) --> cloud
Orbitals from the potential < ---- with the CLOUD
ITERATION
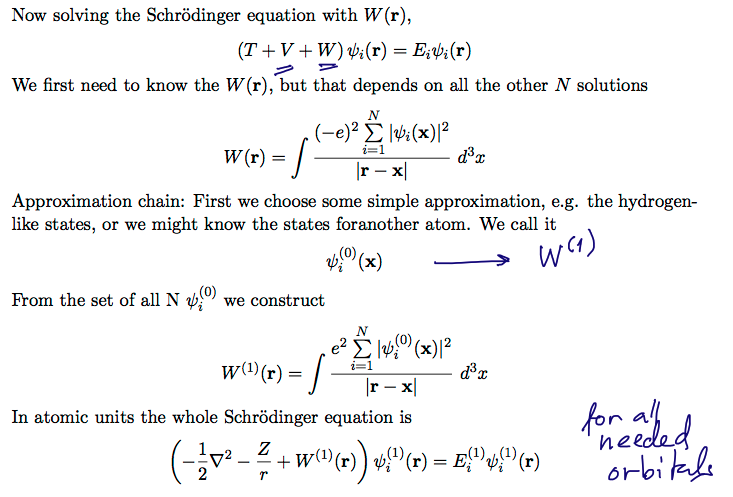
00070_Plug_the_W_into_Schroedinger_Eq.png
ITERATION
SELF-CONSISTENT criterium
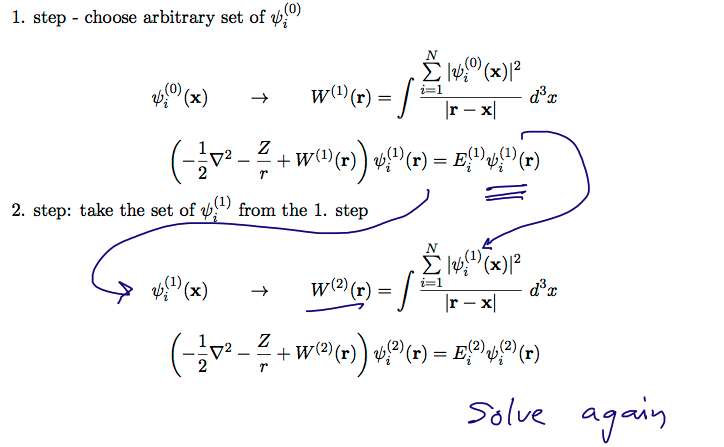
00078_Iteration_selfconsistent_field.png
xcf_0020_orbitals_in_cloud.png
 xcf_0020_orbitals_in_cloud.png |
| ---- COPIED FROM 2014 |
xcf_0025_ORBITALS.png
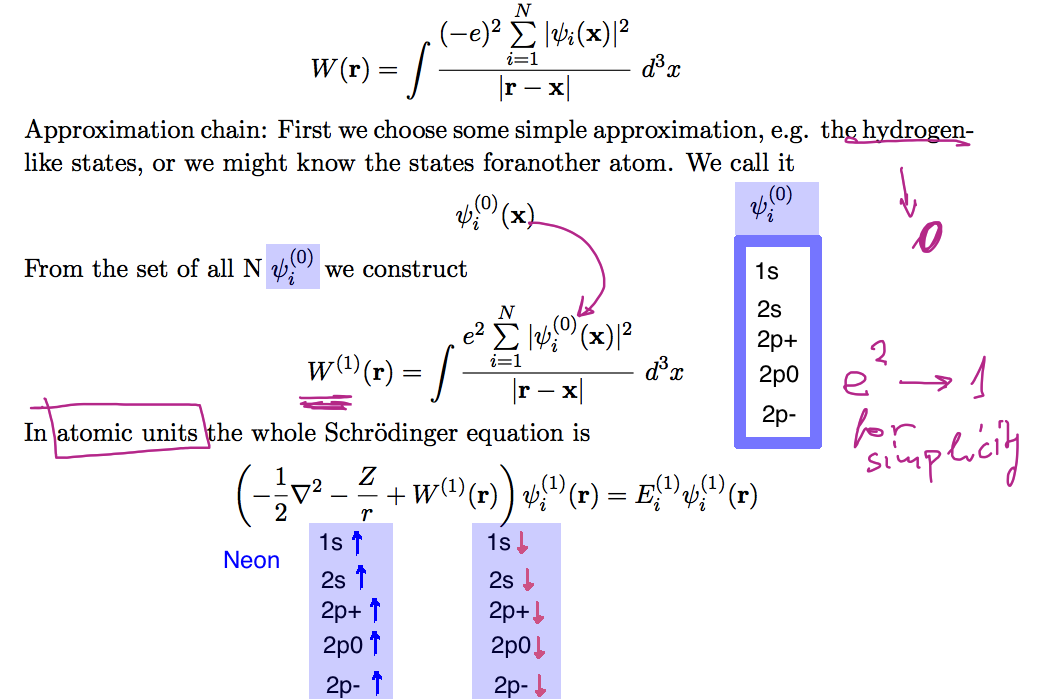 xcf_0025_ORBITALS.png |
Illustration of the iteration; all necessary orbitals must be found ---- COPIED FROM 2014 |
xcf_0029_ITERATIONS.png
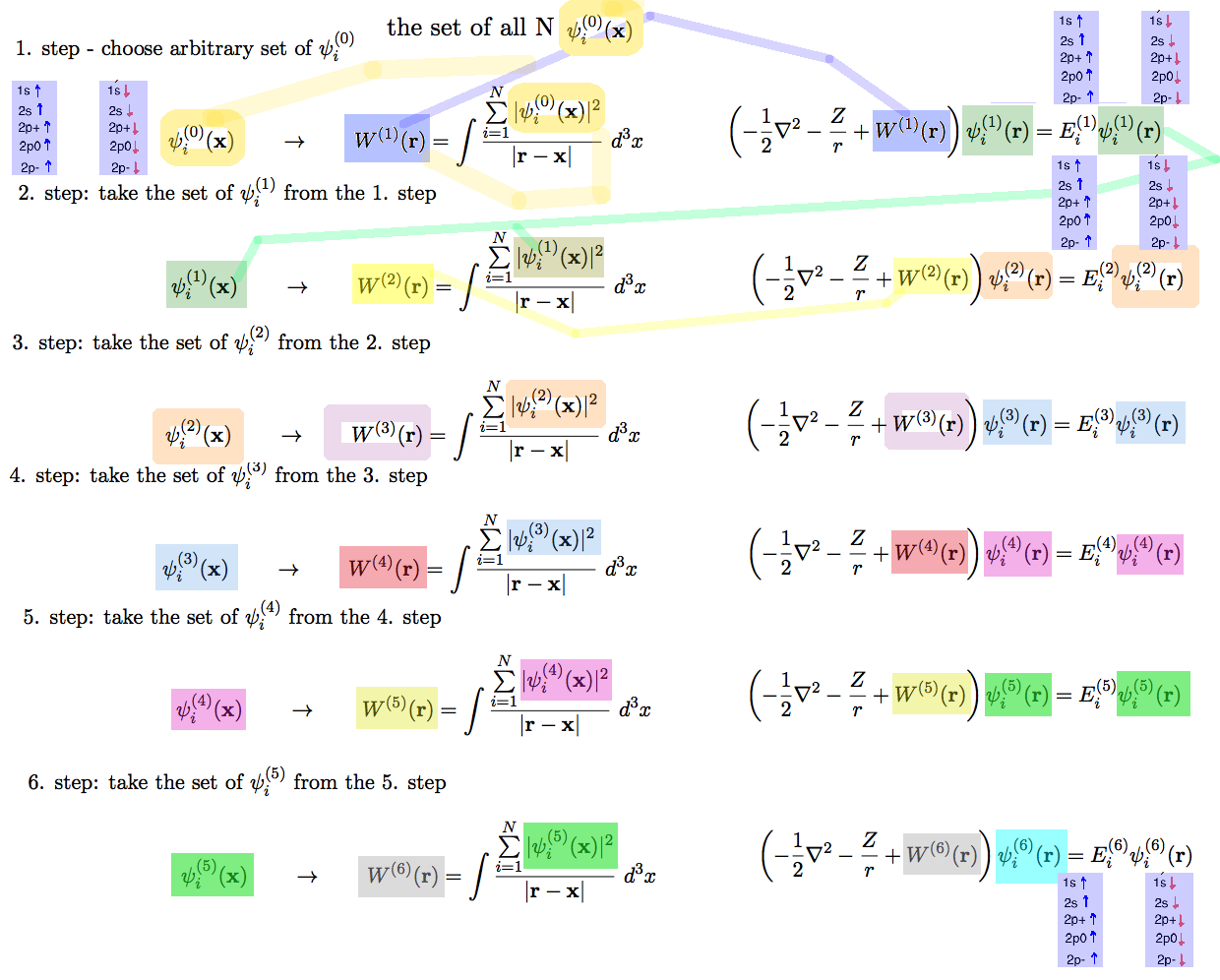 xcf_0029_ITERATIONS.png |
|
----
COPIED
FROM
2014 The iteration in other symbols .... |
xcf_0030_self-Consistent_field.png
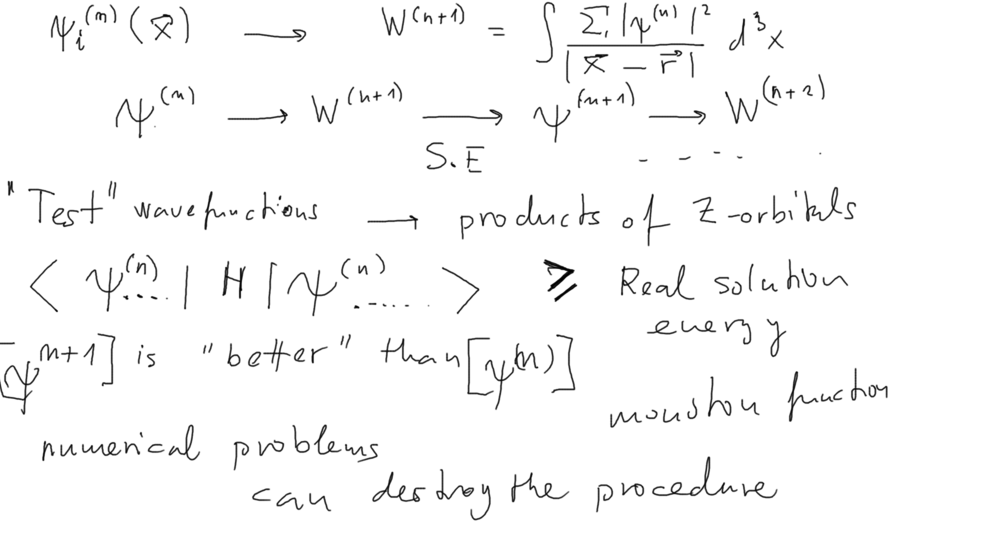 xcf_0030_self-Consistent_field.png |
| SELF-CONSISTENT criterium |
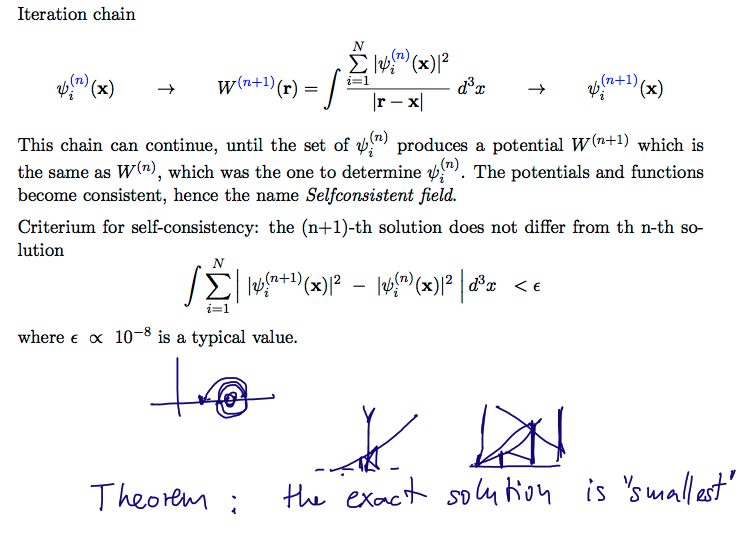
00083_Iteration__Converge-and-stop.png
The above point concludes the introduction of the SELFCONSISTENT FIELD MODEL
---- COPIED FROM 2014
Configurations and energies
Closed shells do not follow the n2 rule 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 4f
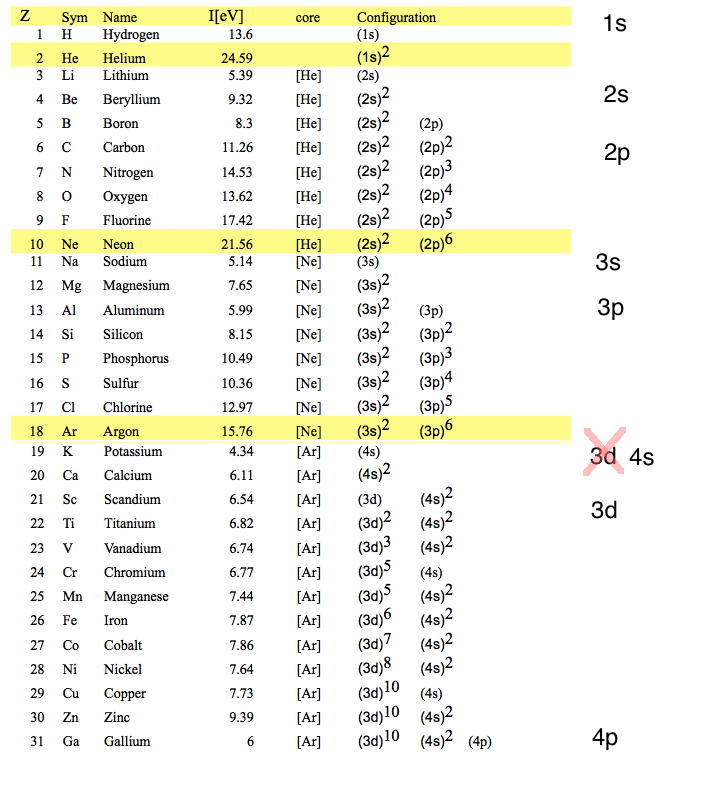
xcf_0040_Configuration_orbitals.png
The explanation of this behaviour - in terms of the self-consitent field - NEXT LECTURE