Introduction - part II - Quantum Physics and the Hydrogen atom
(this version is not yet final; web-links are missing - and some further comments are missing)
Schrödinger equation for the hydrogen atom
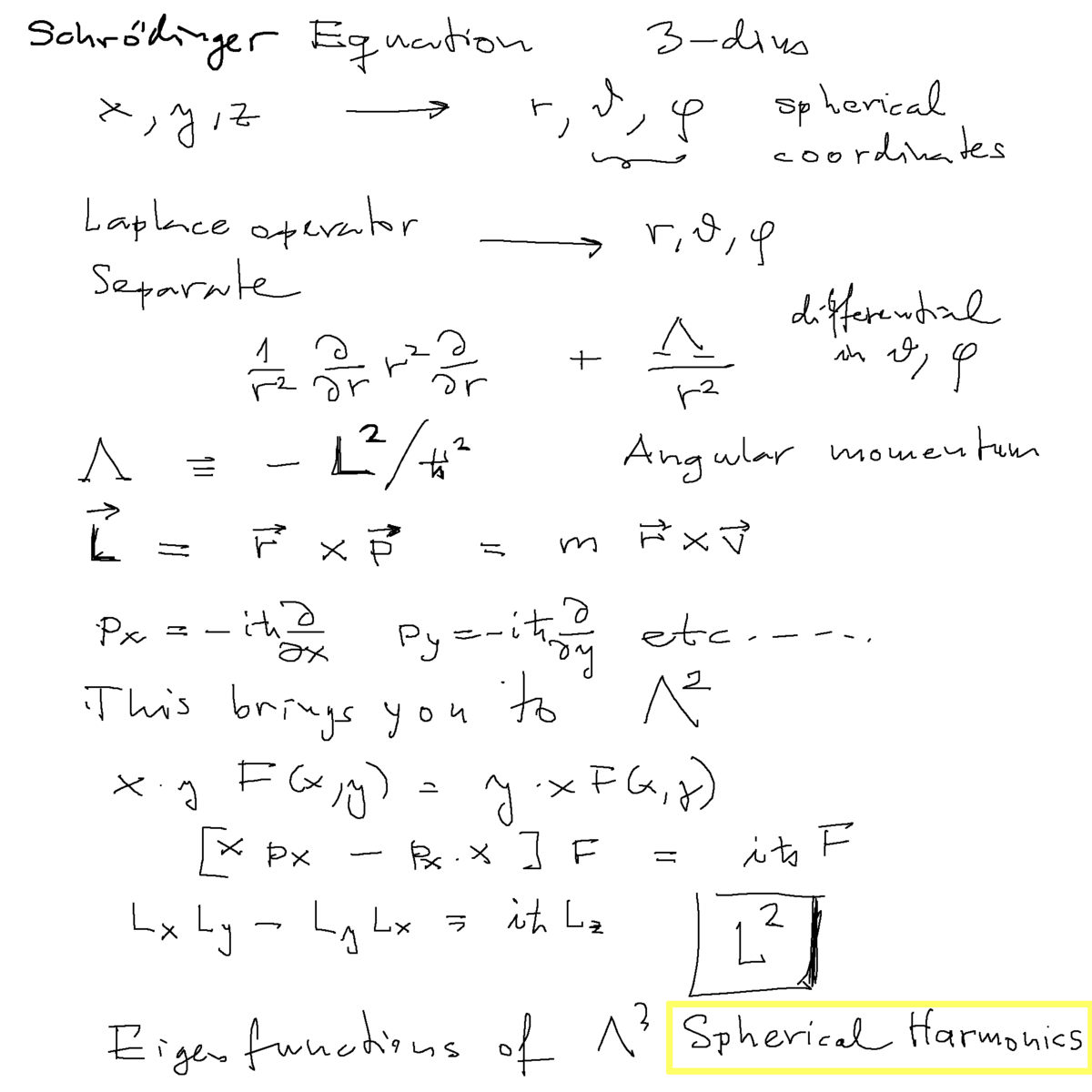
a0010.png
An interesting "daily news" topic - radiative and non-radiative transitions - i.e. how atoms release excitation energy
Autoionizing states in Helium (three lectures ahead)
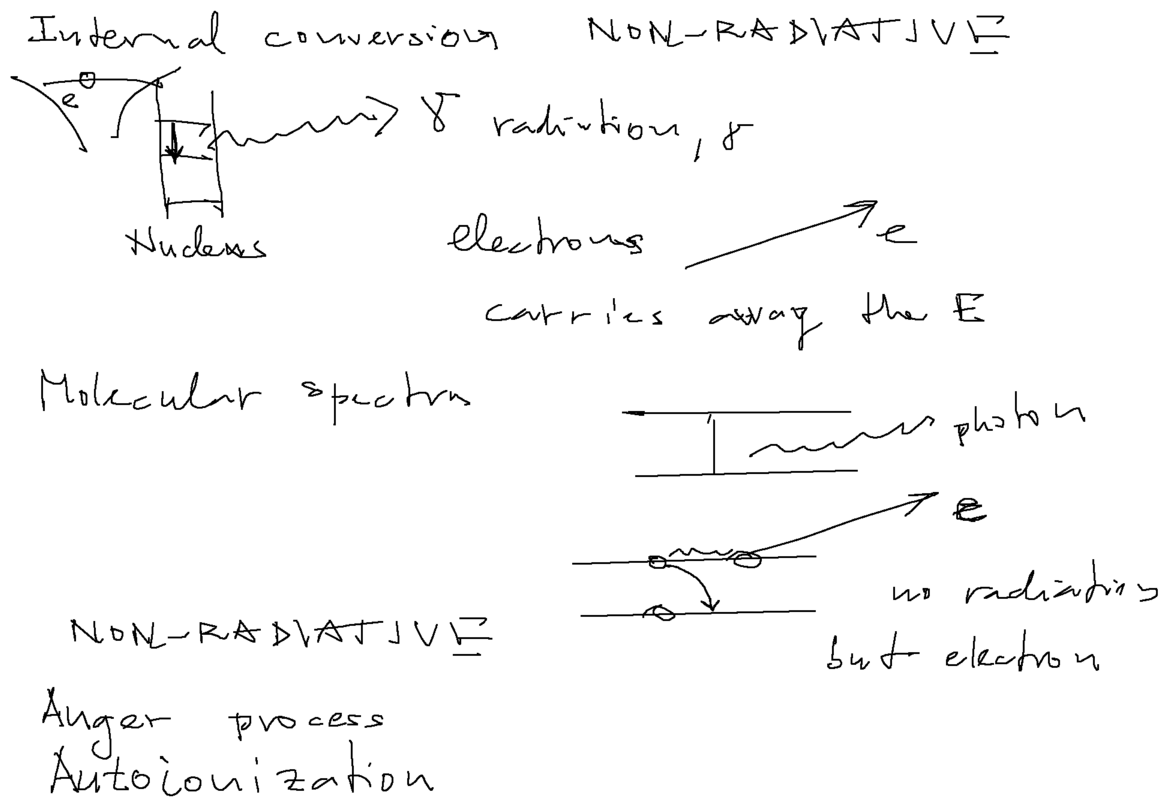
a0020.png
Wavefunctions - Spherical harmonics
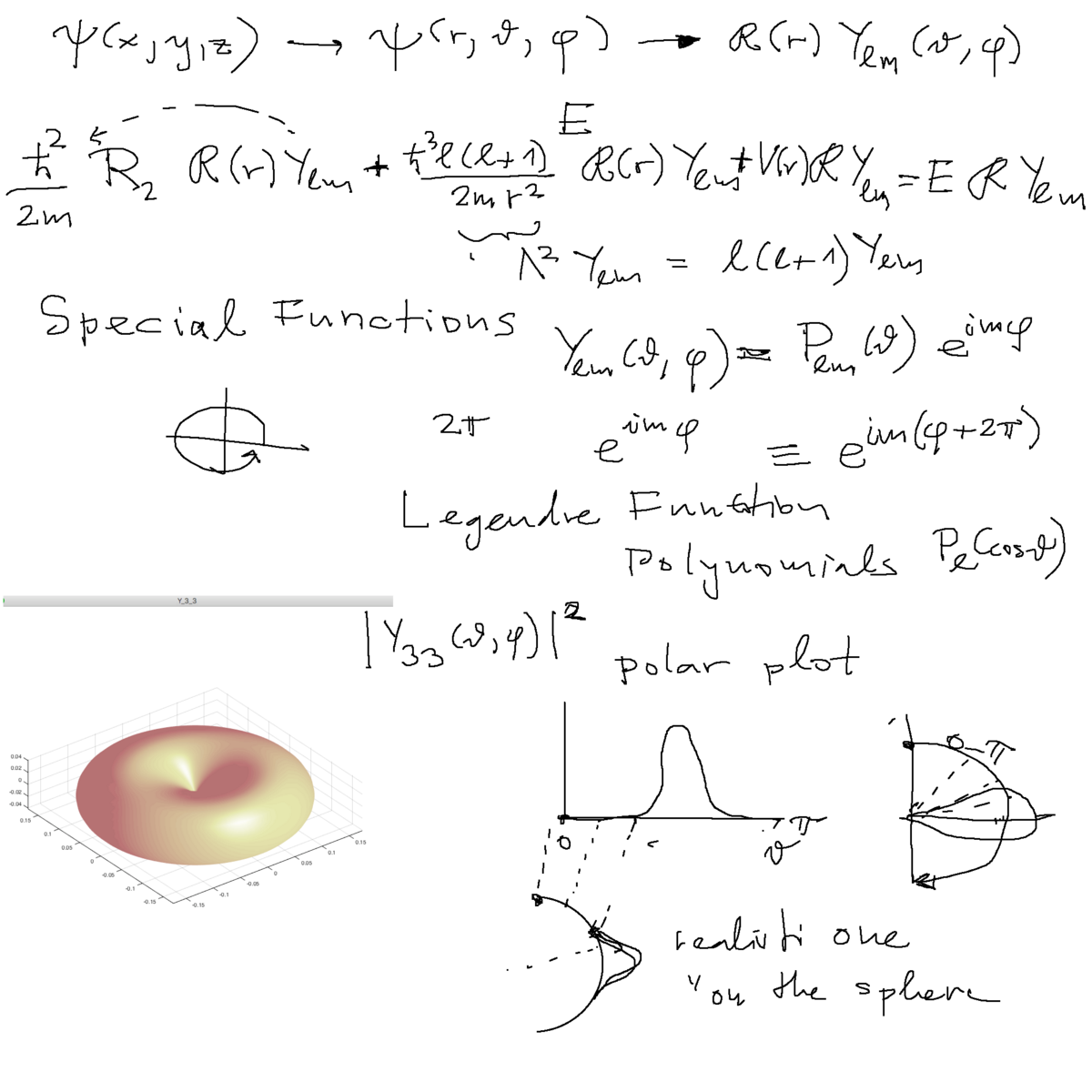
a0030.png
Polar plots; densities ( squared abs. W.F. )
L=6 M=0 L=9 M=4 L=5 M=4
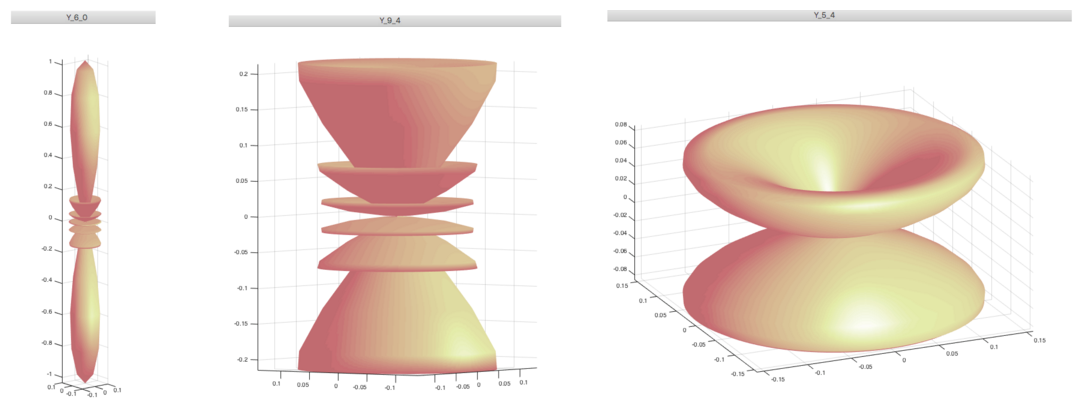
a0040.png
Anatomy of the "orbital wavefunctions" - or rather probability densities
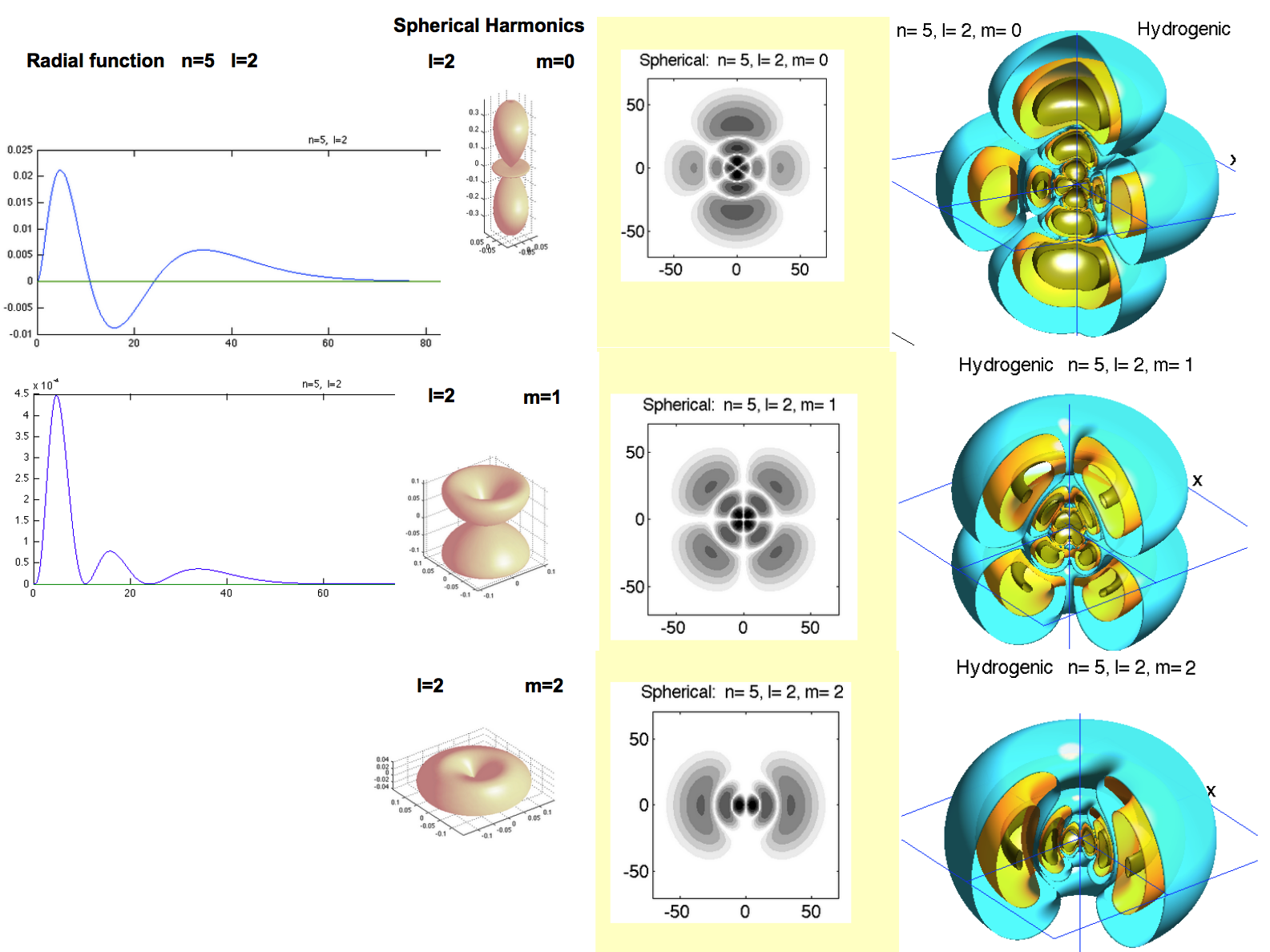
a0043.png
A more dramatic case
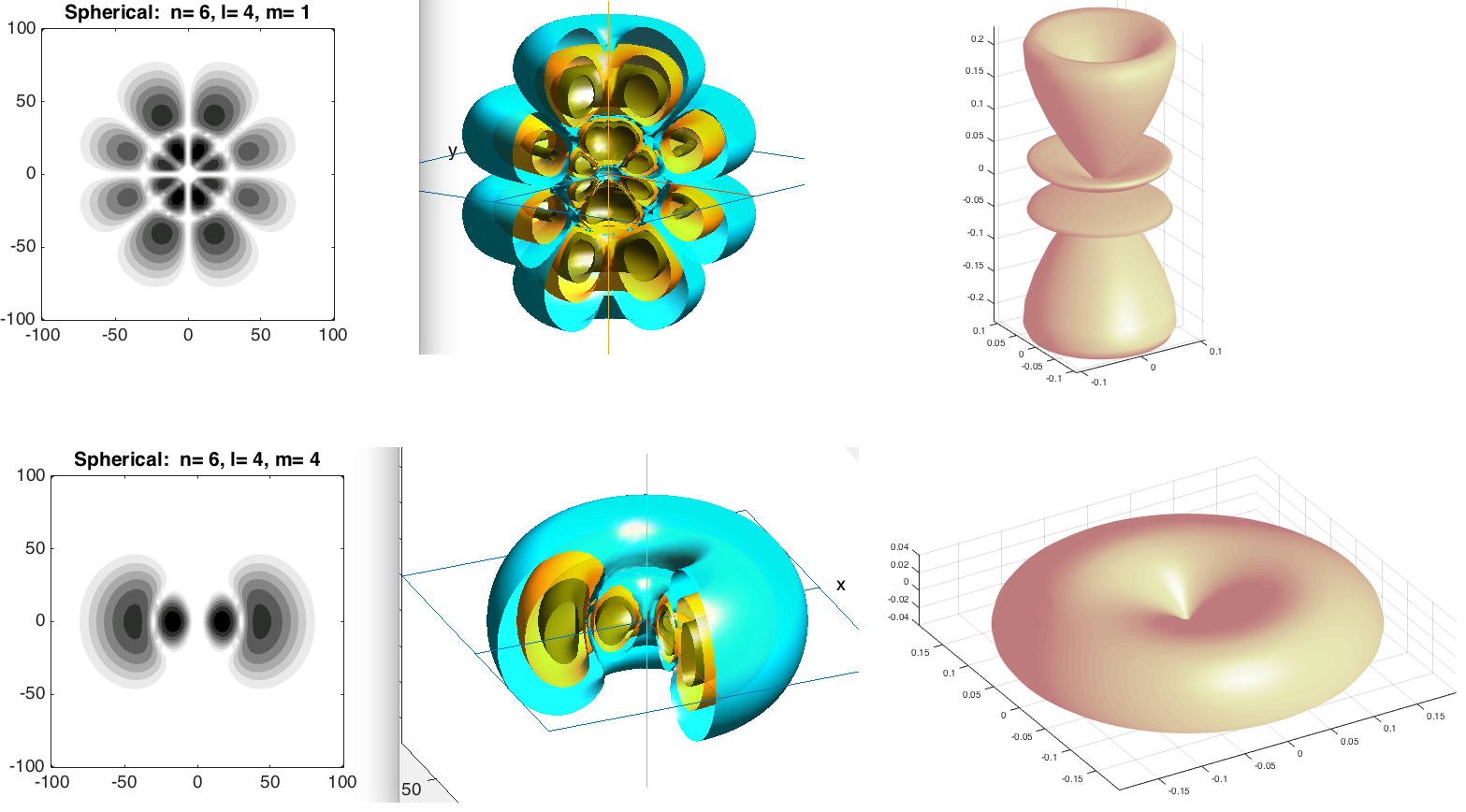
a0045.png
Wavelengths vs energies in electrnvolts - a code The code run in a unix terminal (visible spectrum indicated)
|
Relation of wavelengths - energies - program lines for Octave or MATLAB |
a0050.png 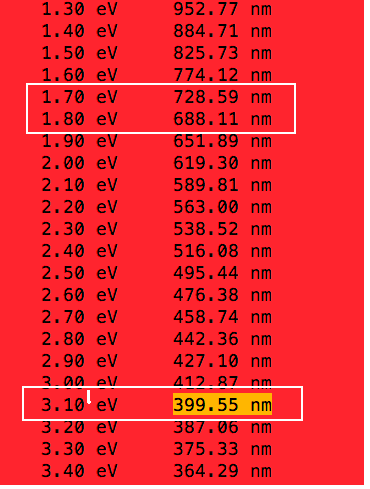 a0050.png |
ATOMIC UNITS
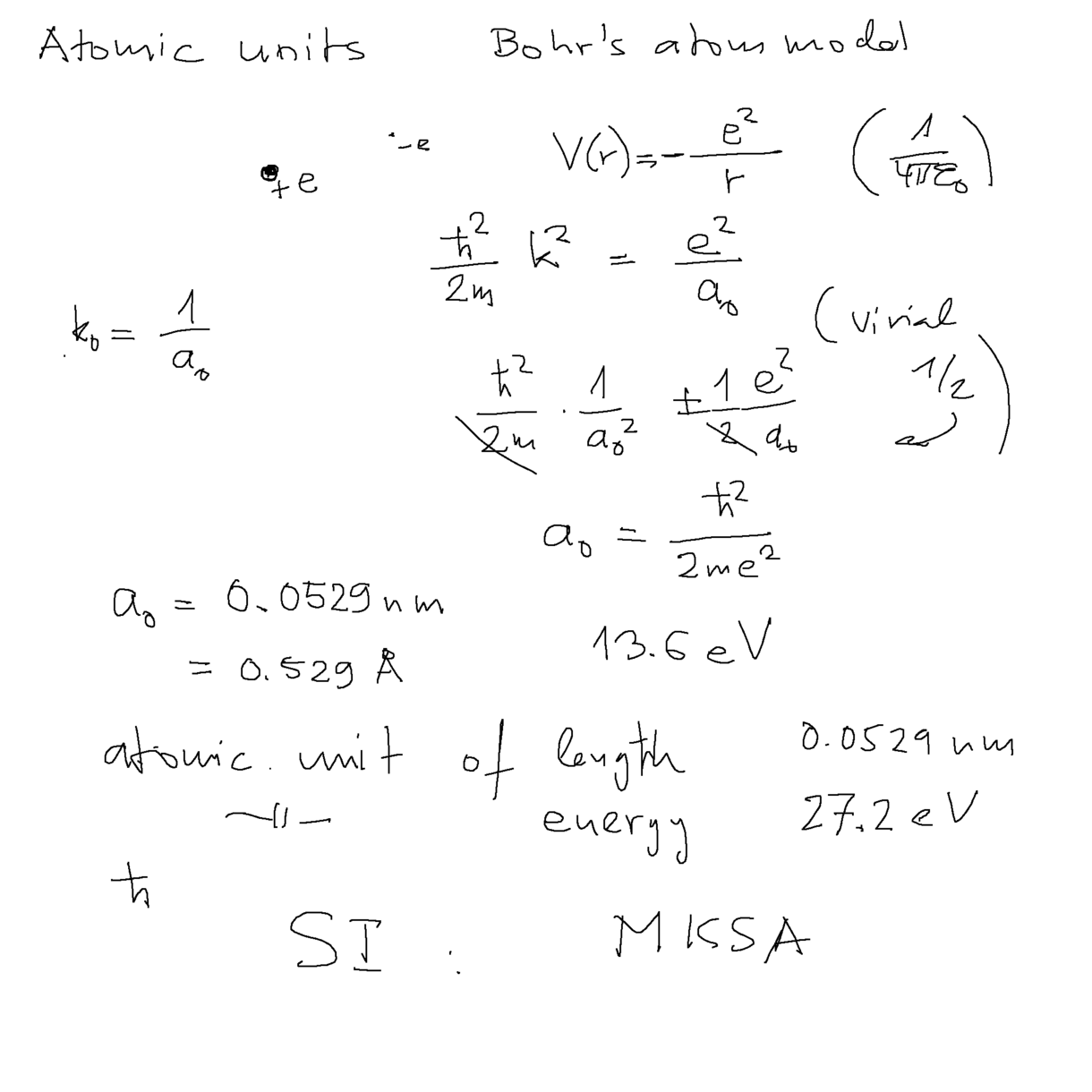
a0060.png
NEXT : introduction part III and The physics of 2-electron atoms