Many-electron atoms - part 1.
Hund's rule - about spin states; in Carbon it is simply the same as triplet
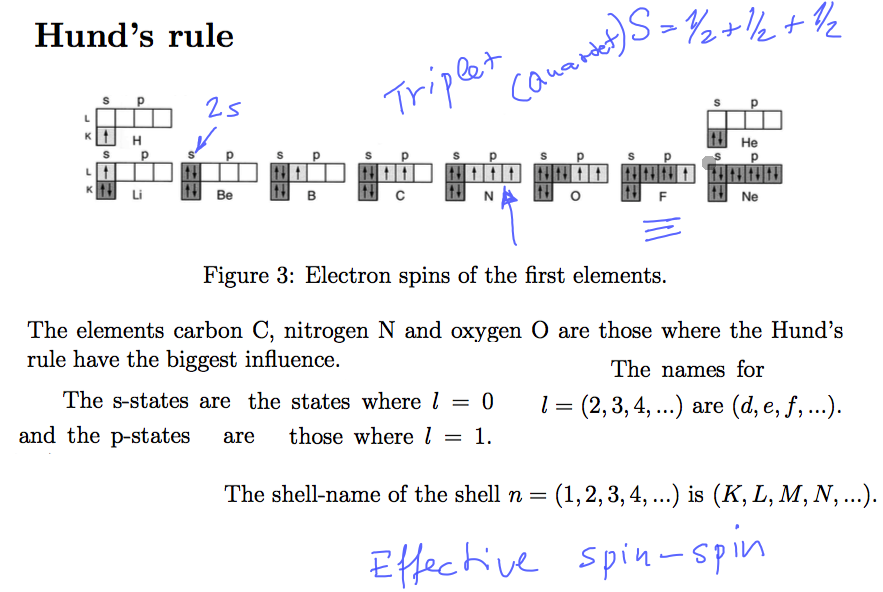
0005_Hunds_rule.png
Number of states in each shell ( n, l, m, ms ) Closed shells - highest ionization energy
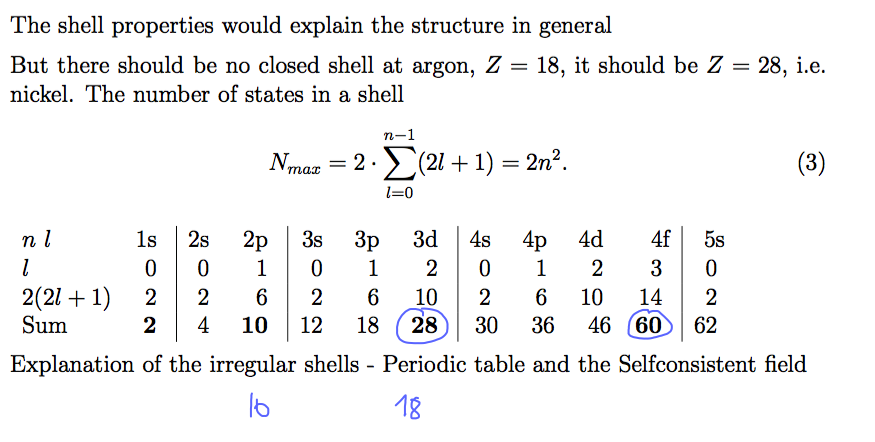
0006_numbers_of_states.png
Closed shells - highest ionization energy - but it does not follow Z= 2, 10, 28, 60, ......
but Z= 2, 10, 18, 36, ......
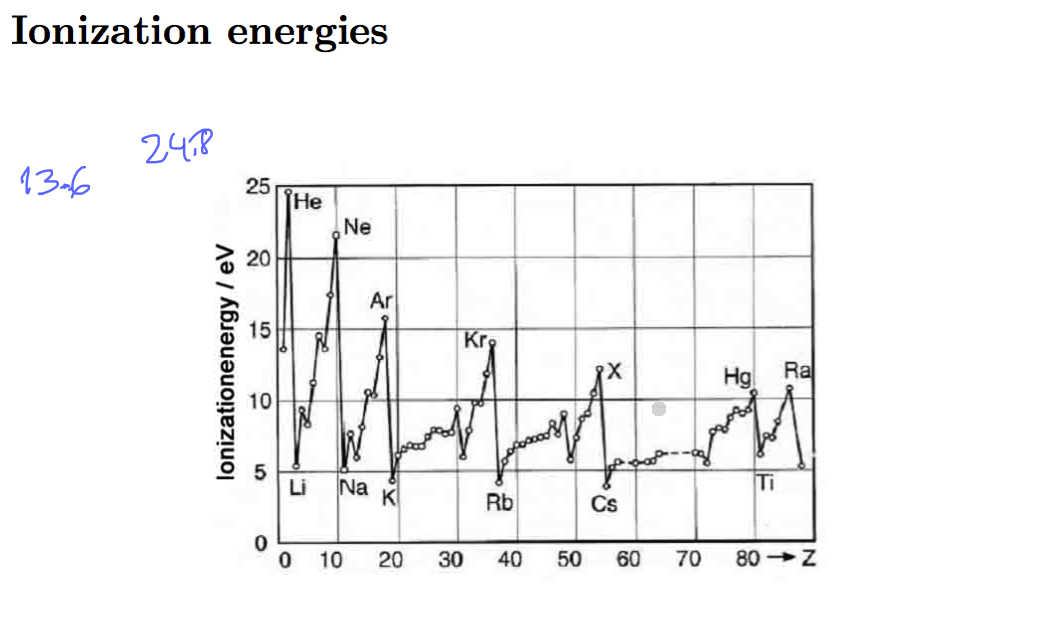
0007_ionization-energies-potentials.png
Here are the first ionization potentials and electron configurations for elements up to 58
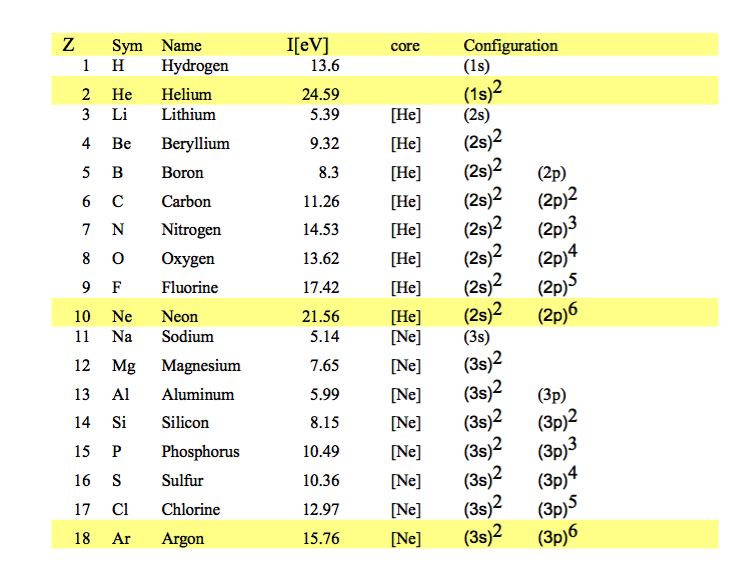
0009_configurations_to_Argon.png
ionization potentials and electron configurations ....
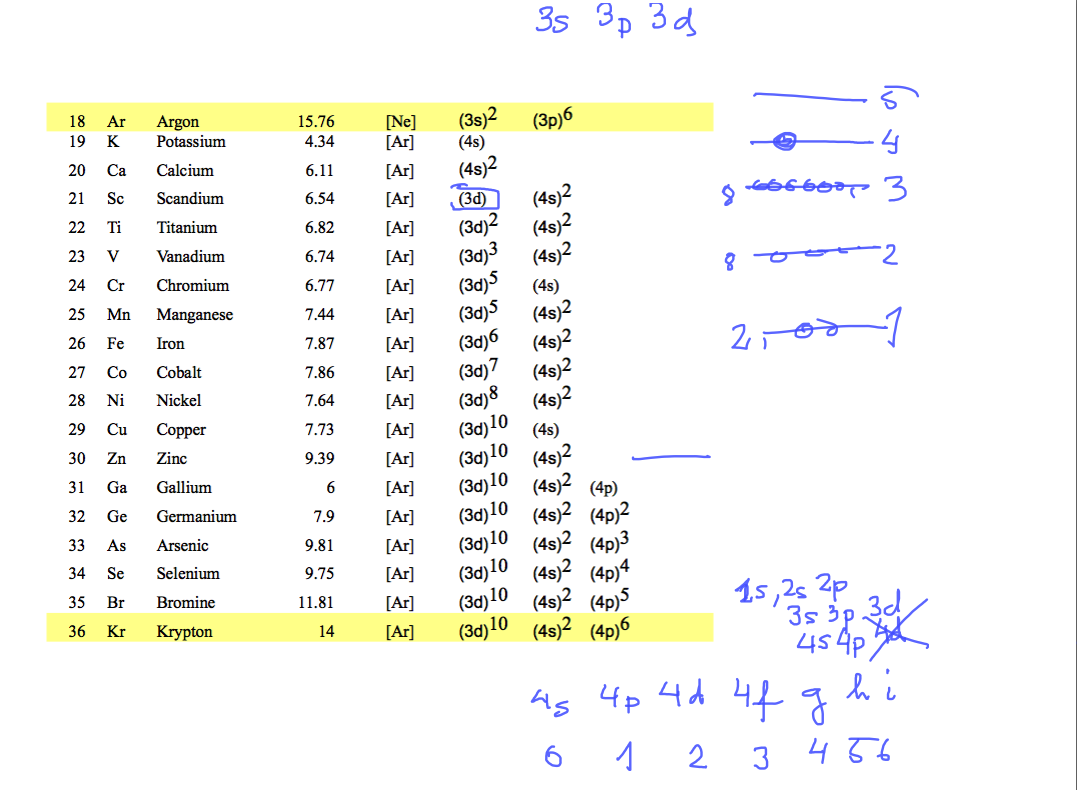
0010_configurations_to_Krypton.png
ionization potentials and electron configurations ....
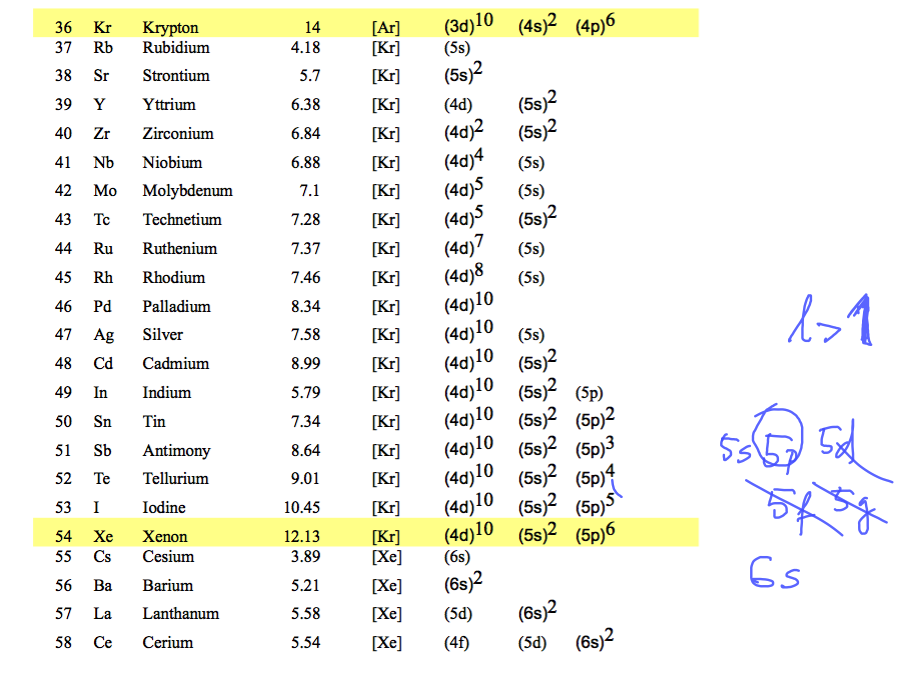
0012__configurations_to_Xenon.png
Why does the 4s fill before 3d? i.e. E4s < E3d E5s < E4d E5s < E4f
Hydrogen like Enl = - Z n -2 E0 independent of L
Because the potential energy of the "last electron" is not the r-1 , not Hydrogen like (see the screening discussion further down)
Radial potentials with added "centrifugal term", i.e. the kinetic energy of rotation, from angular momentum
Coulomb compared with the screened potential - note that Coulomb accomodates the Enl independent of L
Clearly, the screened can not give the same behaviour of Enl - so it must depend on L - and there is a dramatic change
from L=1 to L=2 ( and larger L )
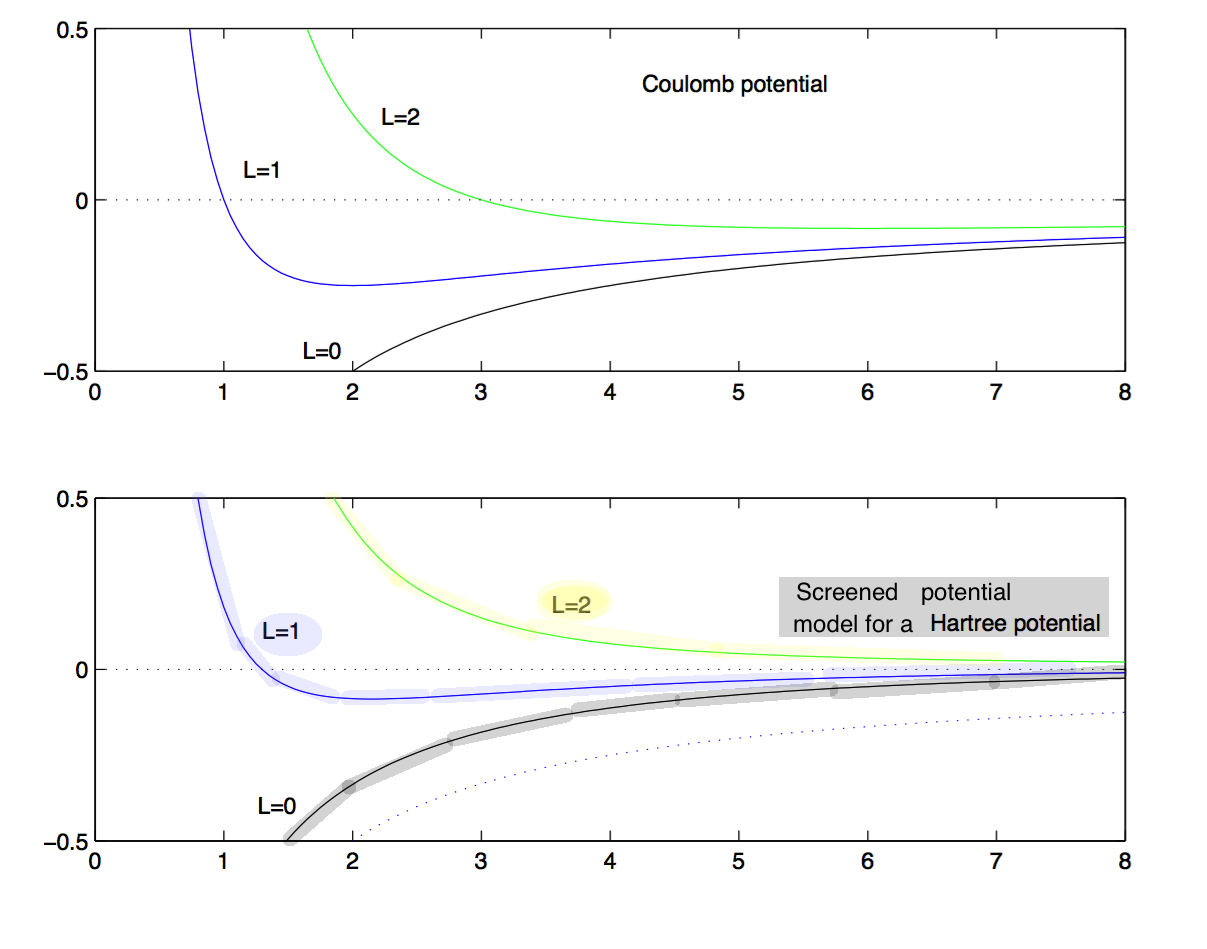
0013_Screened_potential_explains_d-states.png
The above superimposed
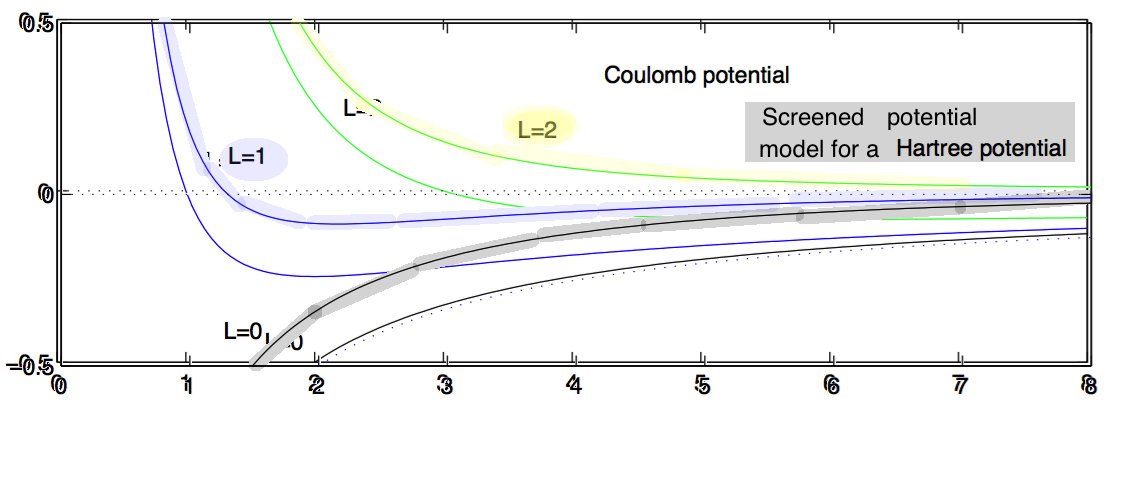
0014_Screened_potential_superimposeds.png
The shape - a model - for the screened potential

0016_Coulomb_Centrifugal_screened.png
The shape - a model - for the screened potential explained. Close to the nucleus - only the Z-charges Coulomb force
Far away - the "last electron" sees only charge 1
the transition - exponential function damping ( model ! )
Also discussed the concept " classical turning point "
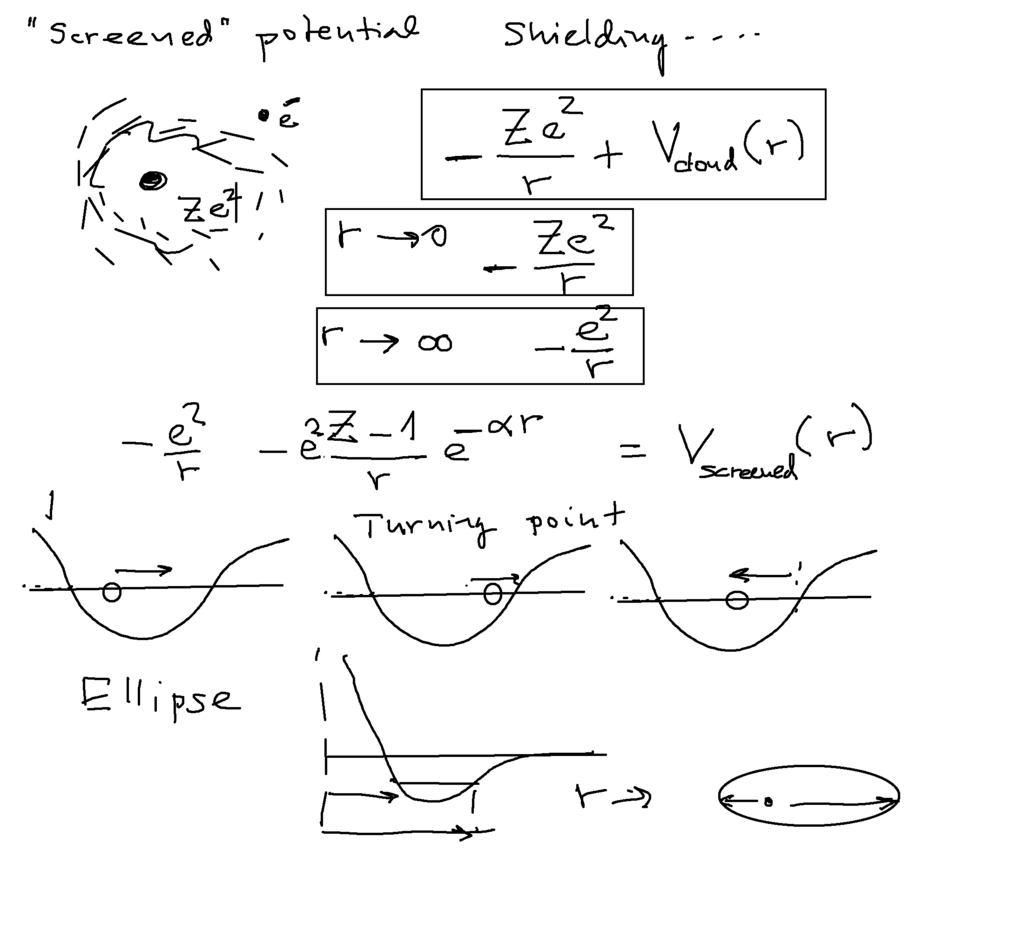
0018_Screened_potential.png
Interaction with a "charged cloud" - towards the selfconsistent potential model
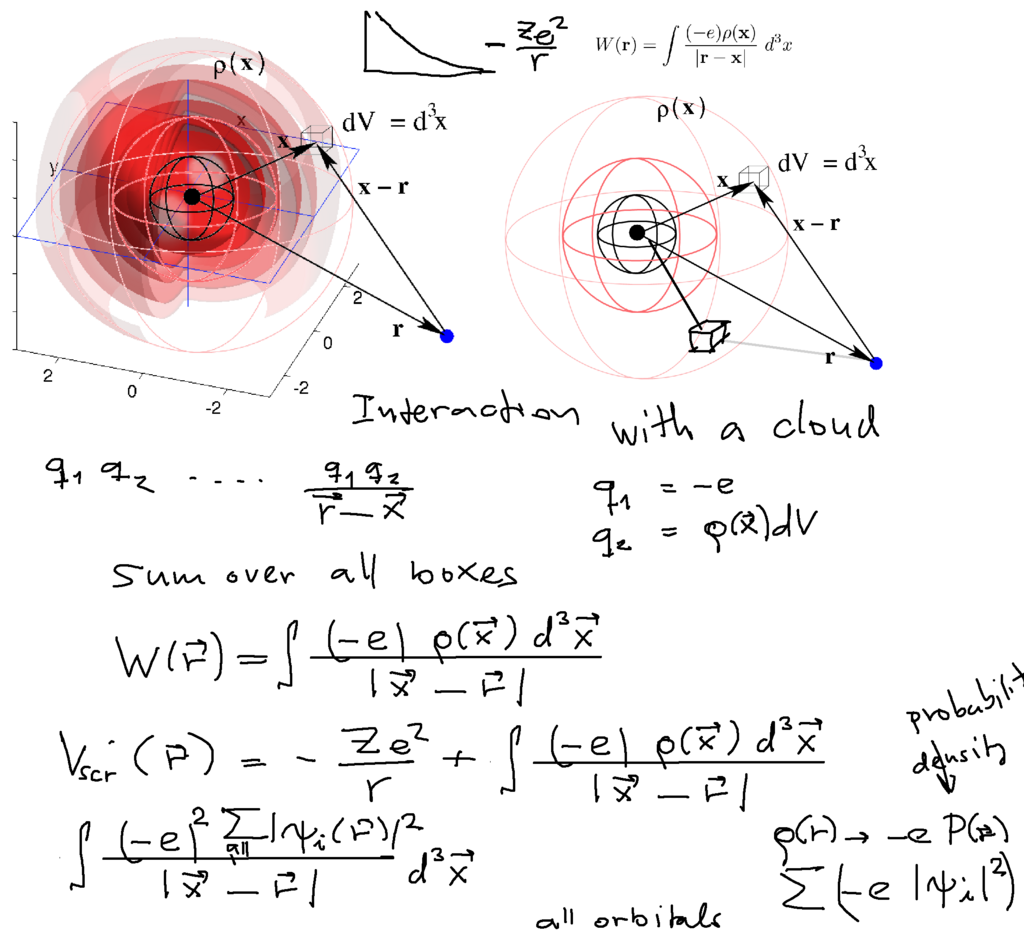
0020_Interaction_with_a_charged_cloud.png
Charged density from probability density - electrons in occupied orbitals
The Schrödinger equation must be solved for obtaining all the orbitals
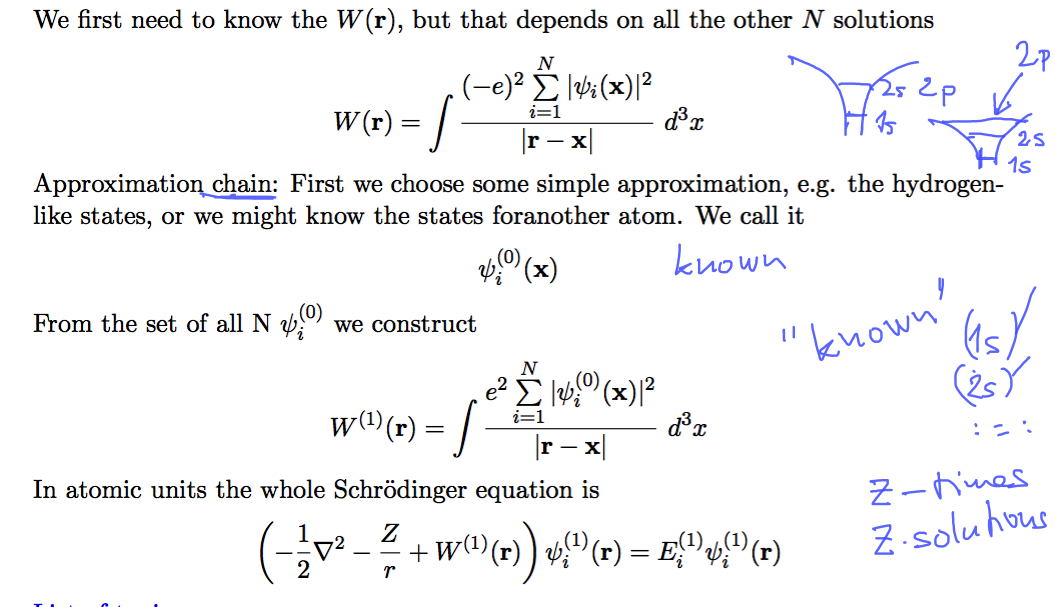
0110_SCF_1.png
Iteration scheme
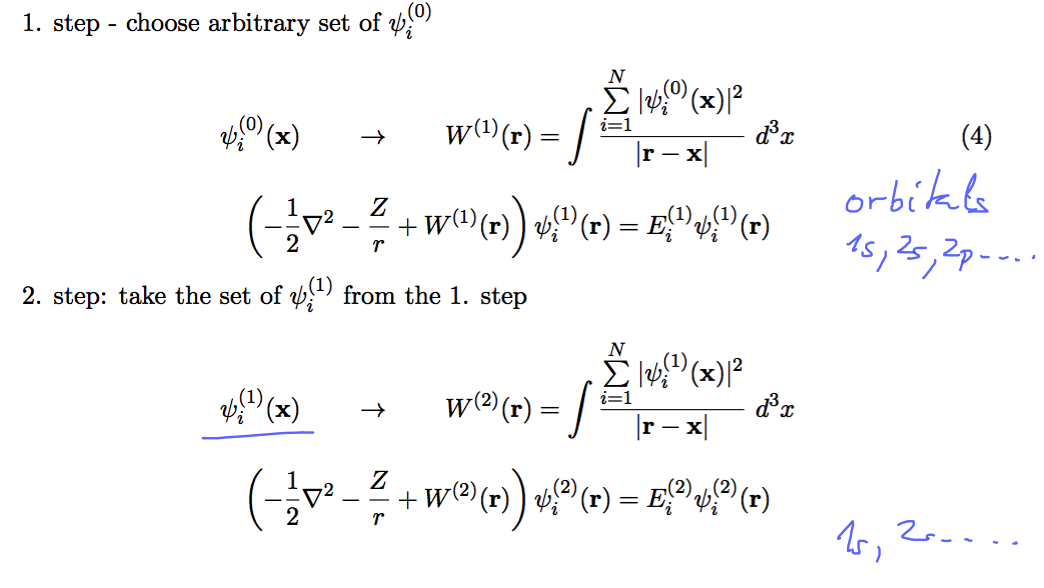
0120_SCF_2.png
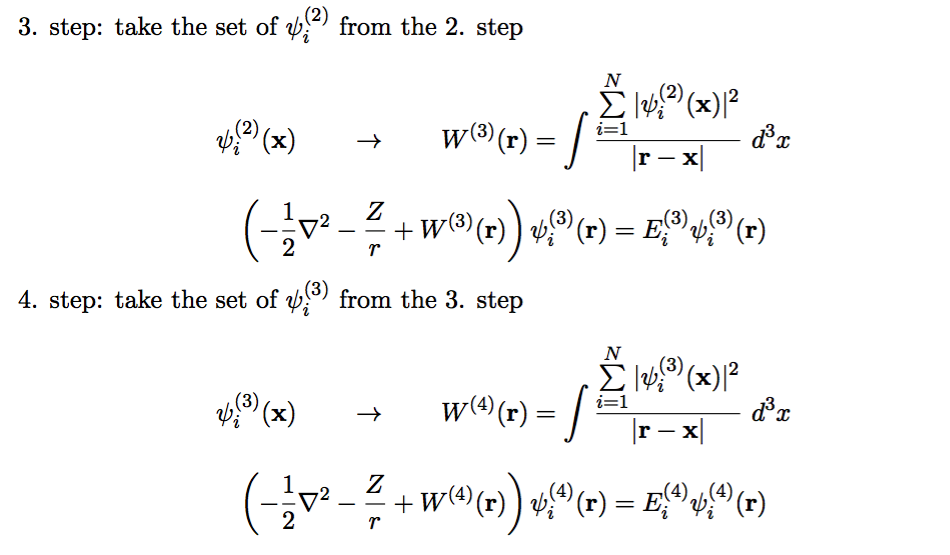
0130_SCF_3.png
Iteration scheme - how to stop it - when the potantial and the orbitals are "self consistent"
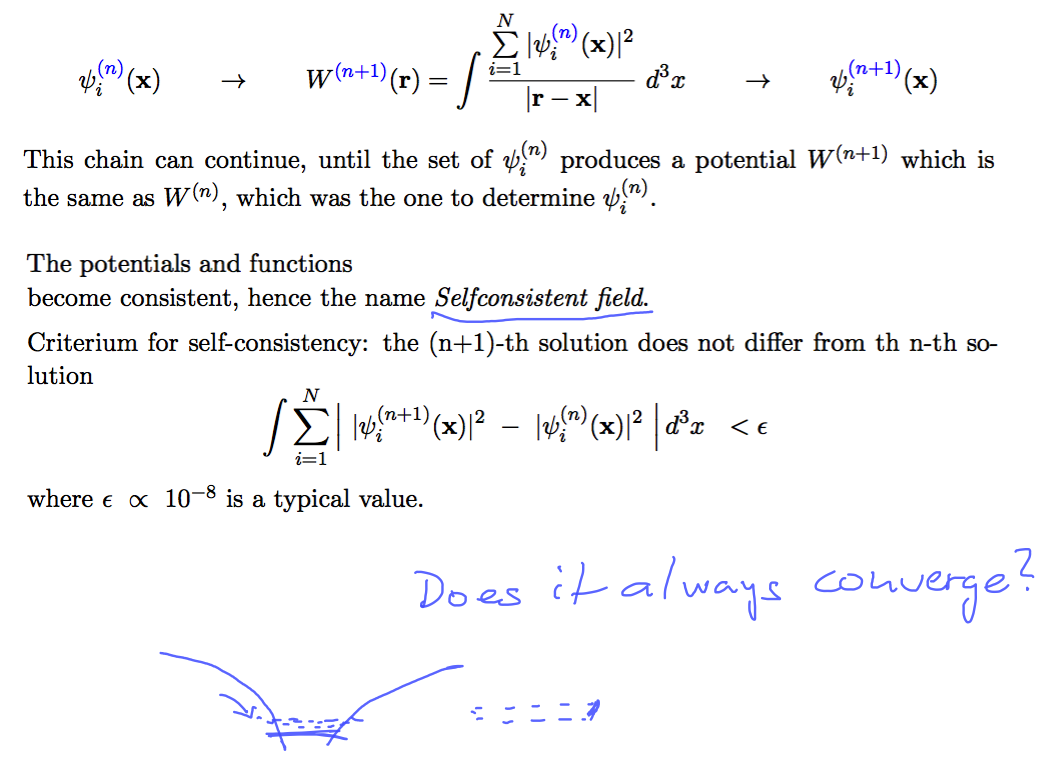
0140_SCF_4.png
The above Hartree - inspired construction is a qualitative and intuitive efficient model - can it be derived precisely?
Formal derivation of many-electron madel - Hartree-Fock method
This starts by evaluation of expectation value for the hamiltonian
Helium N=2
Lithium N=3 - and general N antisymmetric function for N-fermions
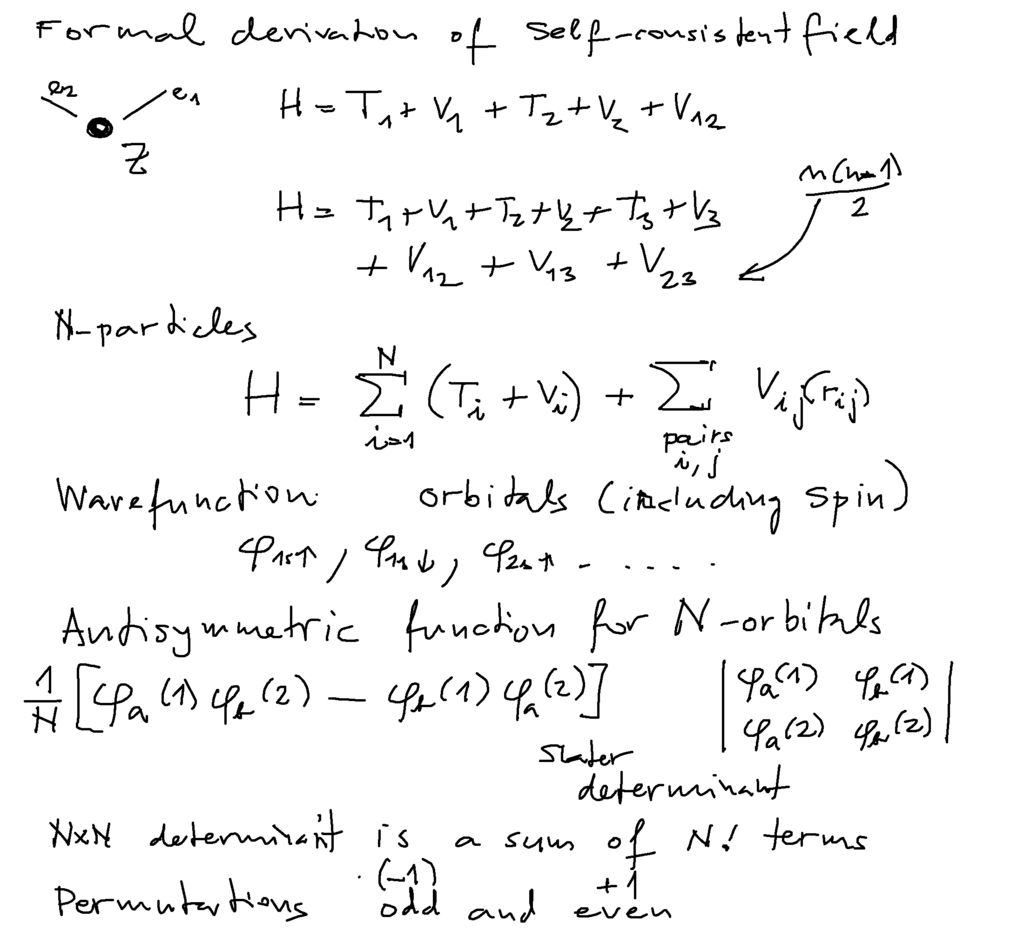
0200_Self_consistent_formal_derivation.png
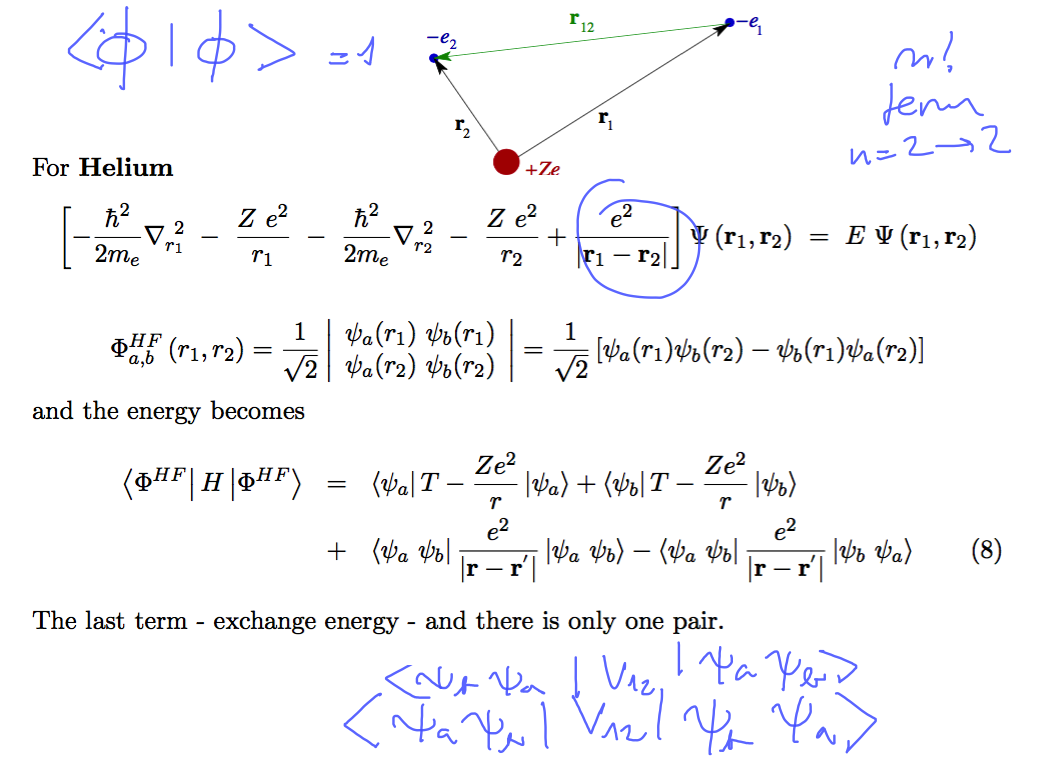
0210_Helium.png
Slater Determinant
PERMUTATIONS, Combinatorics, number of permutations of N objects N!
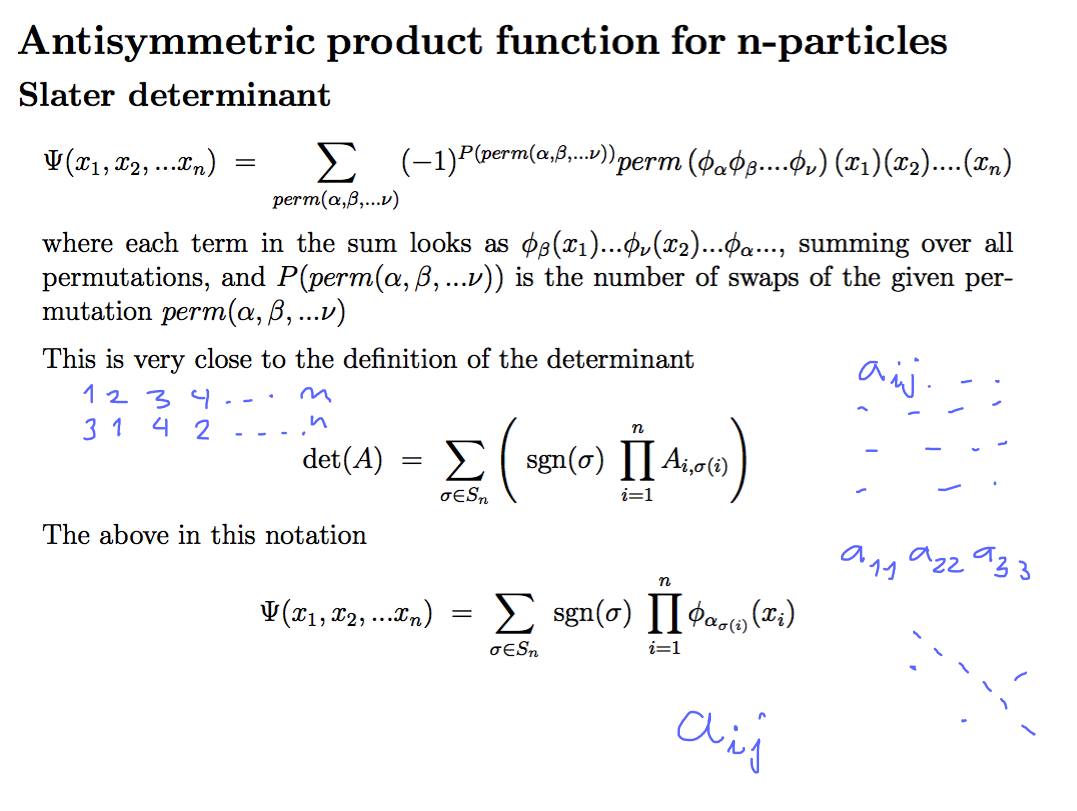
0220_Determinant_Slater.png
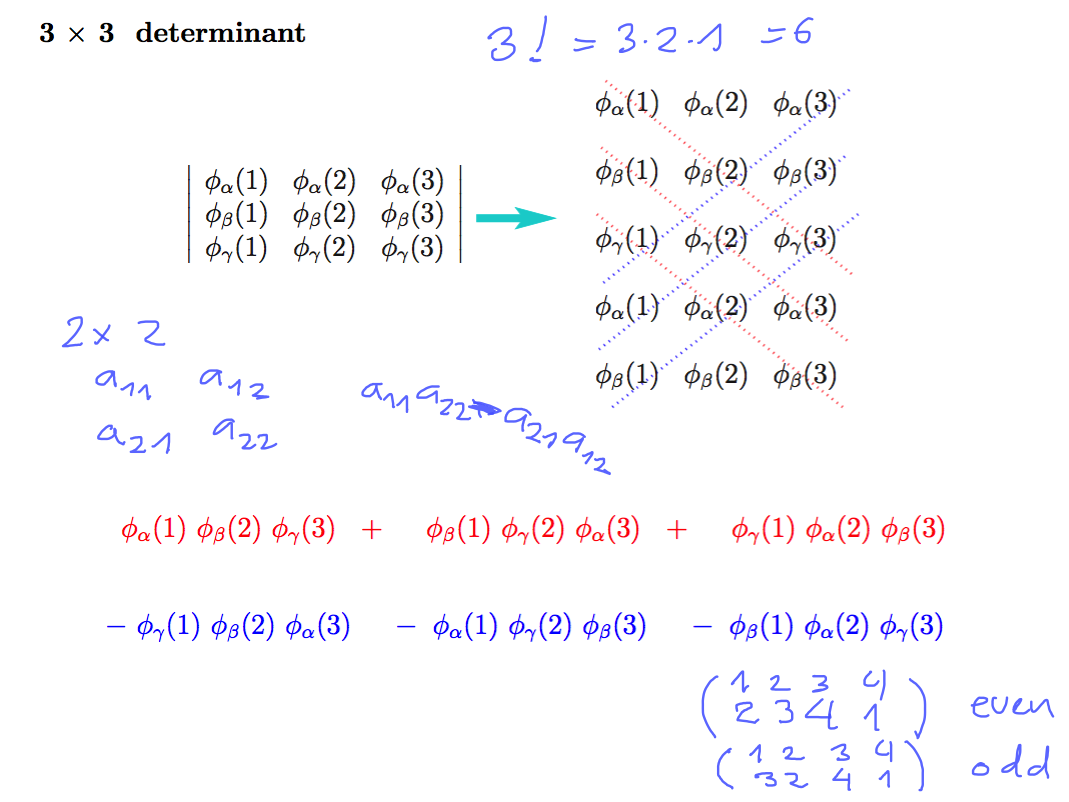
0230_Slater_3x3.png
above - permutations - odd and even
Lithium case - there are many terms (118 pair terms - how to organize them )
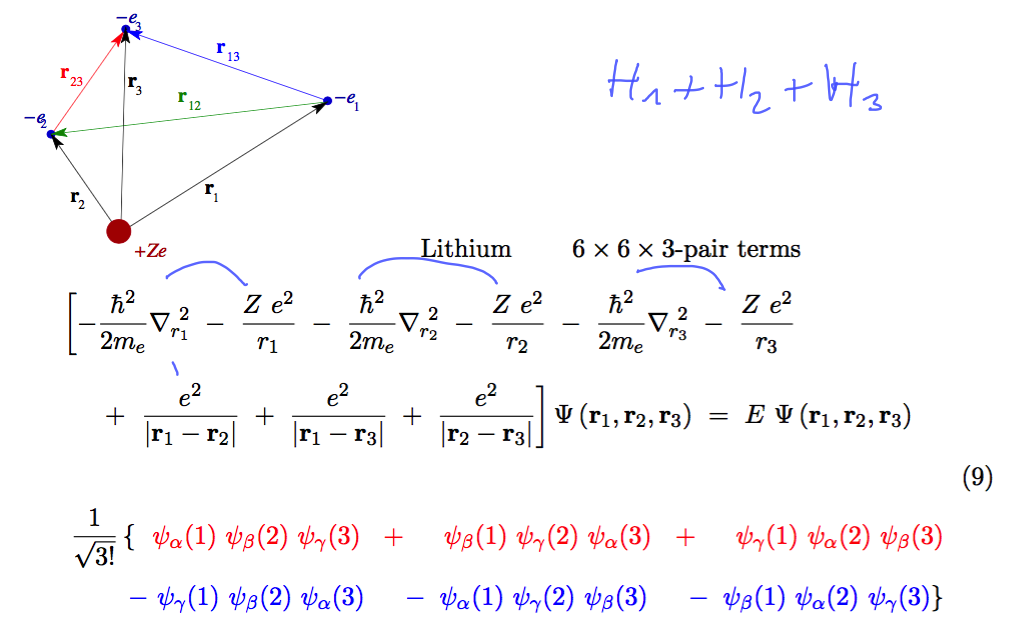
0240_Lithium.png
Next time: Lithium and N > 3 - general case