Spectra - photos of them - Chemistry earlier. But no theory;
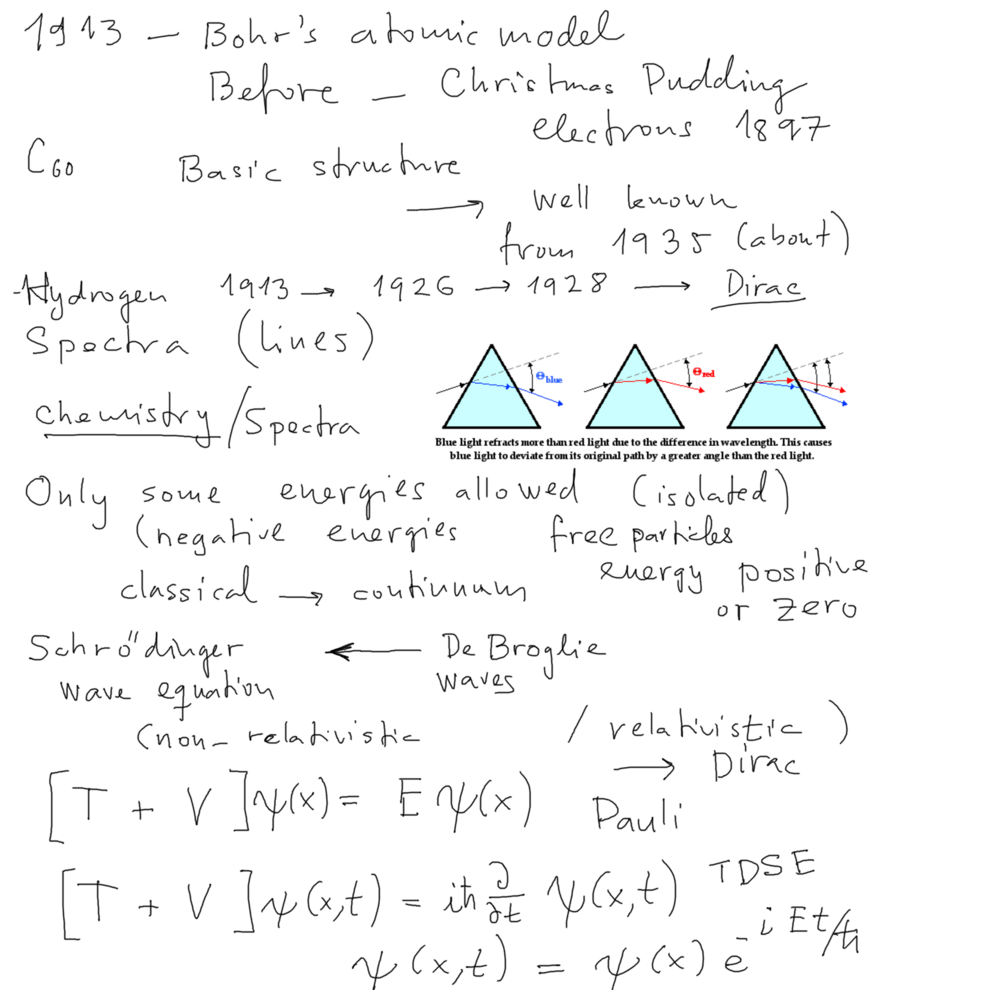
Spectrum - prism - Newton (1704) and before. spectral lines
---- OPTICS - which (visible) color of light moves slowest and which fastest (red is the fast, surprisingly)
---- That is why we have the copied picture (but its text is somewhat crazy - as many things on www are)
Atomic models - Bohr 1913 - before others, J.J. Thompson Christmas pudding ... Look this up
Then we repeated some - oh - so well known "facts" about quantum physics
- bound states (neg. energy) discrete values; lefvels;
- Bohr --> Louis de Broglie --> Schrödinger .... Pauli (Heisenberg a different story ... )
xcf_0000.png
and this "polymorphic" use of Psi - both Psi(x,t) and Psi(x) in the same equation (this is for those who were tortured by C++ prophets)
Next slide:
H = T + V - how did Scrödinger construct the operators? Simplified version sketched below
- based on de Broglie suggestion - probably
what is known for sure - a famous and a very useful DIFFERENTIAL EQUATION results

xcf_0001.png
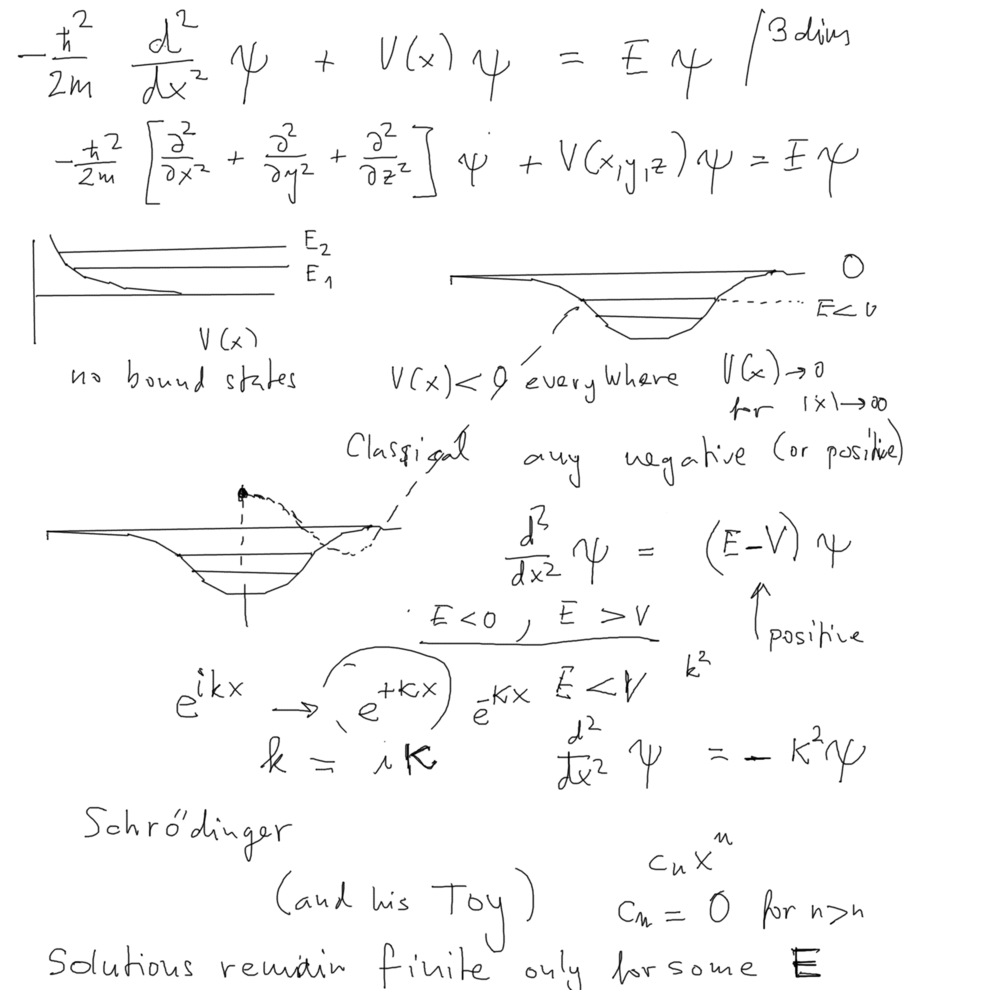
... draw the potentials with and without bound states - JUST AS IN CLASSICAL PHYSICS
only that the bound states can only have "selected energies"
we illustrate why for most n"bound state energies" Schrödinger equation DOES NOT WORK
.... exp (ikx) .... when k behaves rather as k --> + i kappa or k --> - i kappa
.... then exp ( kappa x ) exponential blow-out --- solution nonphysical
.... in textbooks: algebraic methods; power series; only for some values of E infinite series terminated ....
Generally - the dif. eq. simply "loses the tail" for a very special value of energy
Schrödinger's Toy http://web.ift.uib.no/AMOS/schroed/
xcf_0002.png
..... see also the toy in the discussion 2 years ago : ../2013_08_20/index.html
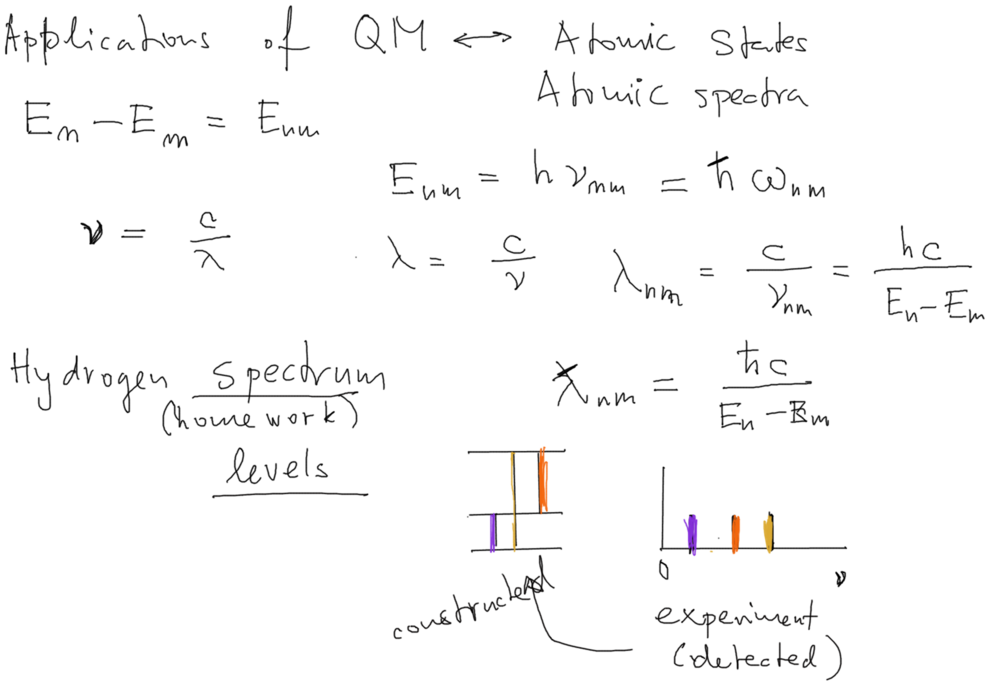
Energy levels and energy spectra
Theory - energy level scheme --> construct transitions lines --> construct spectrum
Experiment - identify line spectra --> construct energy level scheme
h, lambda h-bar, lambda-bar
xcf_0003.png
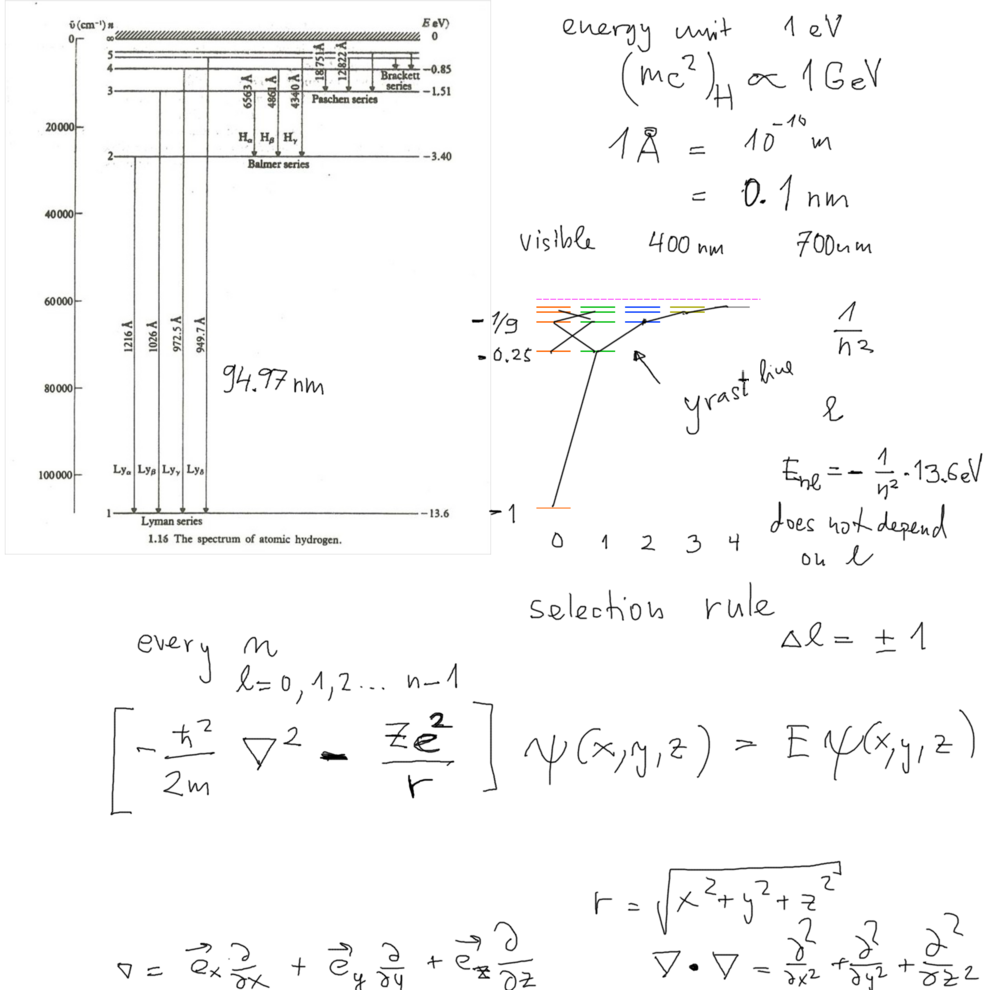
Selection rules -----
xcf_0004.png
yrast line - the one rotating fastest
( yrast = dizziest - Swedish as most of this, Rydberg - but Lyman was American, from Boston, not Swedish)
We shall continue from this equation ....... At the end of the lecture CITIZENDUM - see link below --- (was unresponsive)
see also the whole article http://en.citizendium.org/wiki/hydrogen-like_atom - better than wikipedia
Hydrogen atom wavefunctions http://en.wikipedia.org/wiki/Hydrogen_atom#Wavefunction
Laplace operator http://en.wikipedia.org/wiki/Laplace_operator#Three_dimensions
NEXT LECTURE:
See the above links to http://en.citizendium.org/wiki/hydrogen-like_atom (the whole article)
..... also Graphical study of Hydrogen Bound States (MATLAB)
and the two lectures of the last year: ../2014_08_26/index.html and ../2014_08_21/index.html